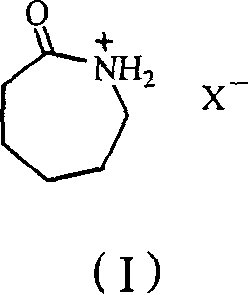
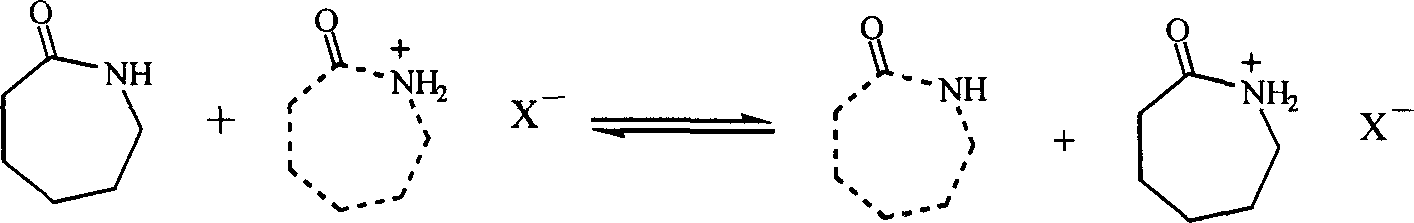
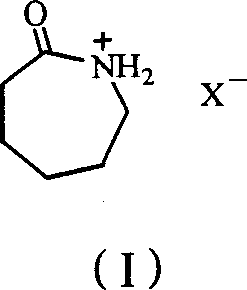
Process for preparing epsilon-hexanolactam by catalyzing cyclohexanone-oxime rearranging
A technology of caprolactam and cyclohexanone oxime, which is applied in the field of catalyzing the rearrangement of cyclohexanone oxime to prepare ε-caprolactam, can solve the problems of acid wastewater pollution, serious equipment corrosion, and low value-added ammonium sulfate, and achieve strong industrial operability Sex, reduce production cost, simplify the effect of the reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take 15mmol of caprolactam fluoroboric acid ionic liquid, put it in a 100ml round bottom flask, add 5mmol of cyclohexanone oxime, gradually raise the temperature to 50°C for 3 hours under stirring, and cool the reactant to room temperature to obtain a homogeneous liquid mixture, take 1ml of it and add 6ml Acetone, fully mixed, chromatographic analysis, the conversion rate is 13%, the selectivity of the product ε-caprolactam is 65%, and the by-product is cyclohexanone.
Embodiment 2
[0026] Take 20mmol of caprolactam fluoroboric acid ionic liquid, put it in a 100ml round bottom flask, add 5mmol of cyclohexanone oxime, gradually raise the temperature to 60°C for 3 hours under stirring, cool the reactant to room temperature to obtain a homogeneous liquid mixture, take 1ml of it and add 6ml Acetone, mixed well, analyzed by gas chromatography, the conversion rate was 61%, and the selectivity of the product ε-caprolactam was 81%.
Embodiment 3
[0028] Take 10mmol of caprolactam fluoroboric acid ionic liquid, put it in a 100ml round bottom flask, add 5mmol of cyclohexanone oxime, gradually raise the temperature to 90°C under stirring and keep it for 6 hours, cool the reactant to room temperature to obtain a homogeneous liquid mixture, take 1ml of it and add 6ml Acetone, mixed well, analyzed by gas chromatography, the conversion rate was 90%, and the selectivity was 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com