Pharmaceutical compound, its preparation method and application in making medicine
A pharmaceutical compound and pharmaceutical technology, applied in the preparation of anticholinergic drugs and drugs for the treatment of respiratory diseases, bromide-1-cyclopropylmethyl-3-quinuclidane pharmaceutical compound and its preparation field, can solve the problem of Accumulation of poisoning in the patient's brain, affecting learning and memory, etc., to achieve long-lasting bronchodilation and protection, relaxation of bladder detrusor, and reduction of medication frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
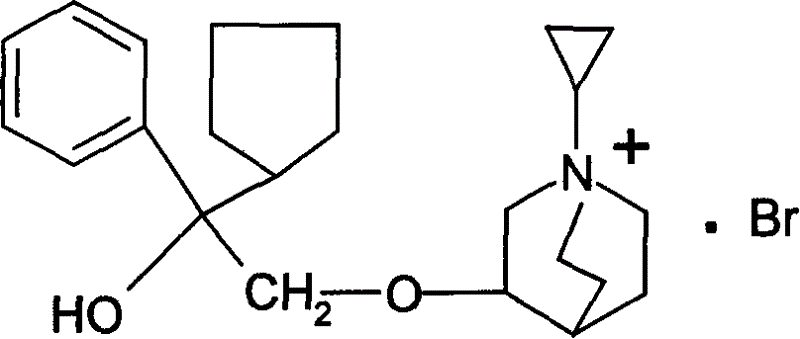
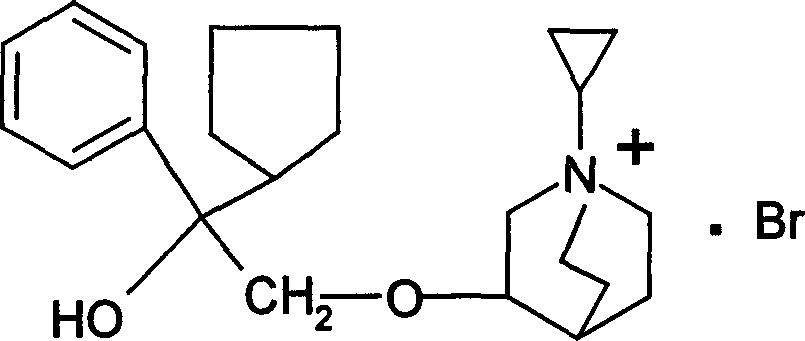
[0026] Preparation of 3-(2-phenyl-2-cyclopentyl-2-hydroxyethoxy)quinuclidane
[0027] In a three-necked reaction flask, weigh 1 g of phenyl-cyclopentyl oxirane and 1.5 g of 3-quinine alcohol, dissolve it in 20 ml of DMSO, add 1 g of sodium hydride, stir and react for 3 hours, cool, extract with ether, and remove the ether layer Extract with 3mol / L hydrochloric acid, neutralize the acid water with 40% NaOH, then extract with ether, dry over anhydrous magnesium sulfate, filter, evaporate the solvent, and the product is purified by distillation for use, with a yield of 57%.
Embodiment 2
[0029] Preparation of 1-cyclopropylmethyl-3-(2-phenyl-2-cyclopentyl-2-hydroxyethoxy)quinuclidine bromide
[0030] Dissolve 5 g of the compound obtained in Example 1 with anhydrous ether, add excess bromomethylcyclopropane, react overnight, pour off the supernatant, and recrystallize the remaining solid with acetone to obtain a white solid with a yield of 67%. The melting point measured by YRT-3 melting point apparatus is 153-154°C.
Embodiment 3
[0032] Antispasmodic effect of 1-cyclopropylmethyl-3-(2-phenyl-2-cyclopentyl-2-hydroxyethoxy)quinuclidine bromide on isolated guinea pig bronchi
[0033] Guinea pigs were killed by bloodletting from the femoral artery after violently hitting the head, and the trachea from the larynx to the bifurcation of the trachea was quickly taken out, placed in a petri dish filled with Krebson’s solution, and the trachea was cut into 20×3cm 2 Fix the lower end of the helical strip of the trachea to the bottom of the McFarland bath filled with 15ml of Krebson's solution, and a thin line at the upper end is connected to the tension transducer, which is connected to the second physiological recorder, and the tracheal load is adjusted to 1.5 ~2g, and feed 95% oxygen and 5% carbon dioxide gas, keep the temperature at 37 degrees, and stabilize for 60-90 minutes. Add different concentrations of Ach into the bath by cumulative dosing method, record the shrinkage amplitude of each concentration, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com