Chinese traditional medicine composition for promoting immunity and preparation and quality controlling method thereof
A quality control method and composition technology, which can be used in drug combinations, testing drug preparations, and pharmaceutical formulations, etc., can solve problems such as side effects, and achieve the effects of stable preparations and controllable quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0038] Experimental example 1: Extraction process test
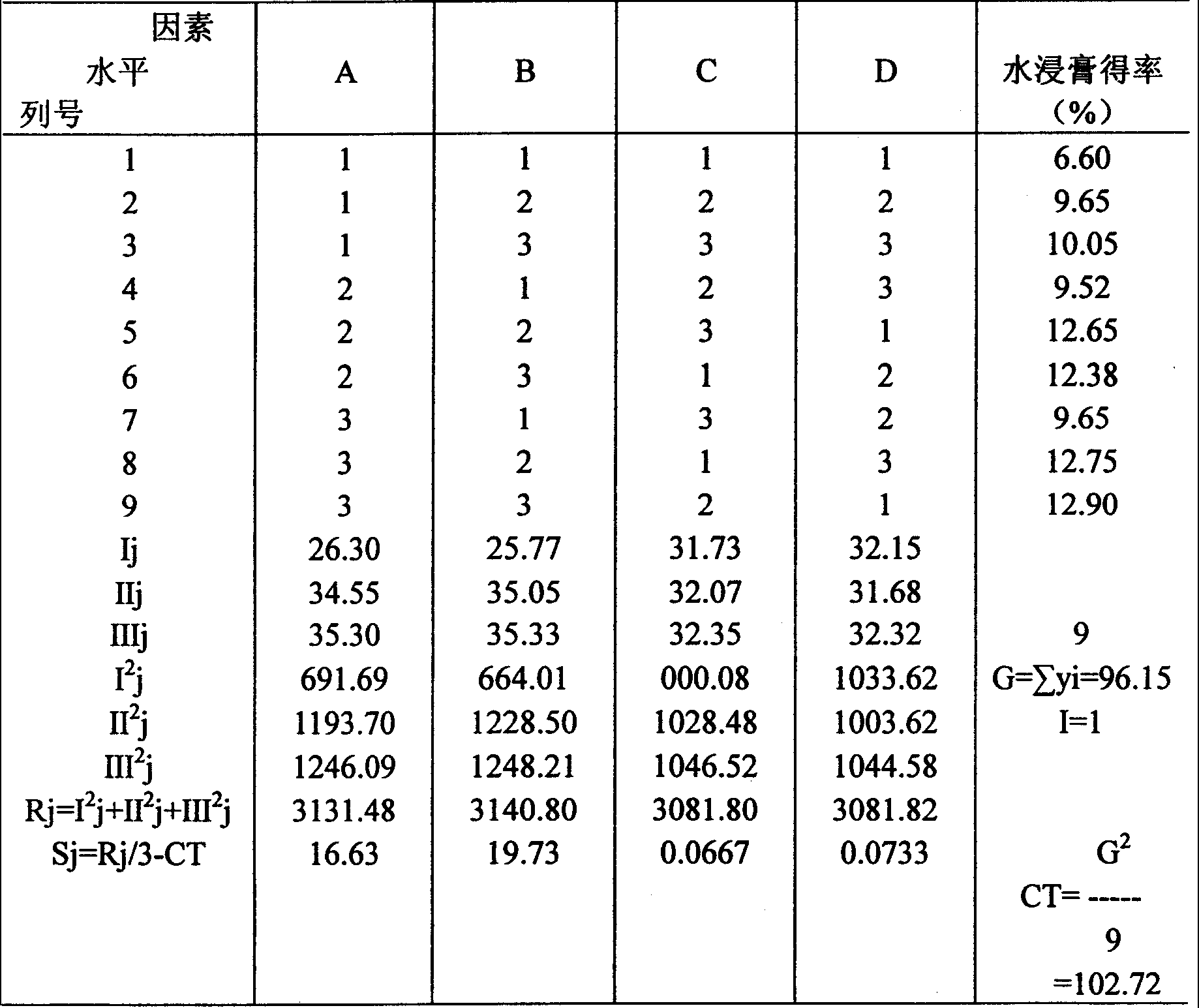
[0039] According to the traditional decoction method of the decoction, decoct the herbal flavor together with boiling water twice, each time for 1 hour, and use the method of centrifugation and standing to remove water-insoluble impurities. Select three factors such as decoction time, decoction frequency and decoction water consumption, select three levels of each factor, select 0.75-1.5 hours for decoction time, select 1-3 times for decoction times, select 5-3 times through pre-test 8 times the amount of water added is more appropriate. See Table 1 for factor levels.
[0040]
[0041] The three factors were arranged in the L9(34) orthogonal table, and the variance analysis was carried out. The index of investigation was the water extract determined by the drying method. The yield results are shown in Table 2 and Table 3.
[0042] Table 2 Orthogonal Design Table L9(34)
[0043]
[0044] source of...
experiment example 2
[0049] Experimental Example 2: Examination of the drying temperature of the extract and auxiliary materials (see Table 6)
[0050] temperature(℃)
[0051] Experiments show that the content does not change much when the drying temperature is 60°C and 80°C, and it is advisable to choose a lower drying temperature of 60°C based on other factors.
experiment example 3
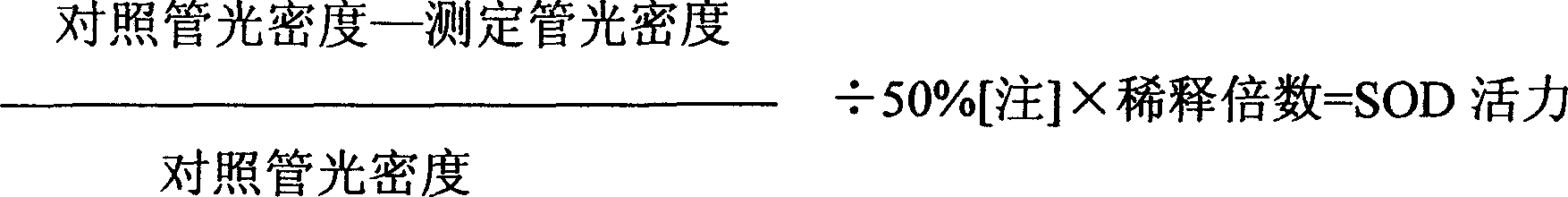
[0052] Experimental Example 3: Content Determination Test
[0053] Take 10g of the sample, weigh it accurately, place it in a Soxhlet extractor, add 80ml of methanol to reflux and extract until the extract is colorless, recover the methanol from the extract and concentrate it to dryness, add 10ml of water to the residue, dissolve it with slight heat, and use water-saturated n-butane Alcohol shaking extraction 3 times, 15ml each time, combined n-butanol extract. Recover the solvent under reduced pressure, add 25ml of water to the residue to dissolve in stages (10, 5, 5, 5), pass through the D101 macroporous resin column (inner diameter 1.5cm, length 10cm) successively, add 20ml of 0.5mol / L sodium hydroxide solution to wash, Rinse with water until neutral, then elute with 50ml of 40% ethanol, discard the 40% ethanol eluate, and finally elute with 80ml of 70% ethanol, collect the eluate, evaporate to dryness, dissolve the residue in methanol and transfer to 1ml In the volumetric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com