Composition for lowering serum uric acid level
A technology for serum uric acid and composition, applied in the field of lactic acid bacteria and yeast strains, can solve the problems of weakening food taste, difficulty in accurately limiting purine intake, impossible to follow for a long time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0362] (1) Experiment of the purine decomposition ability of the microorganism of the present invention
[0363] The purine decomposing ability of the microorganisms of the present invention was evaluated according to the following method.
[0364] (1-a) Microbes of the present invention (ONRIC b0185 (FERM BP-10004), ONRICb0193 (FERM BP-10005), ONRIC b0195 (FERM BP-10006) and ONRICb0223 (FERM BP-10007)) and used for anaerobic culture An oxygen adsorbent (AnaeroPack, manufactured by Mitsubishi Gas Chemical Co., Ltd.) was placed in a sealed container and anaerobically cultured at 28° C. for 48 hours using MRS medium for lactic acid bacteria strains and YM medium for yeast strains. After culturing, microorganisms were collected by centrifuging the culture solution at 3000 rpm for 10 minutes at 4°C. Two ml of a 0.1 M potassium phosphate solution (pH 7.0) containing inosine and guanosine at a concentration of 1.25 mM was added to each microorganism thus cultured. The microbial su...
Embodiment 2
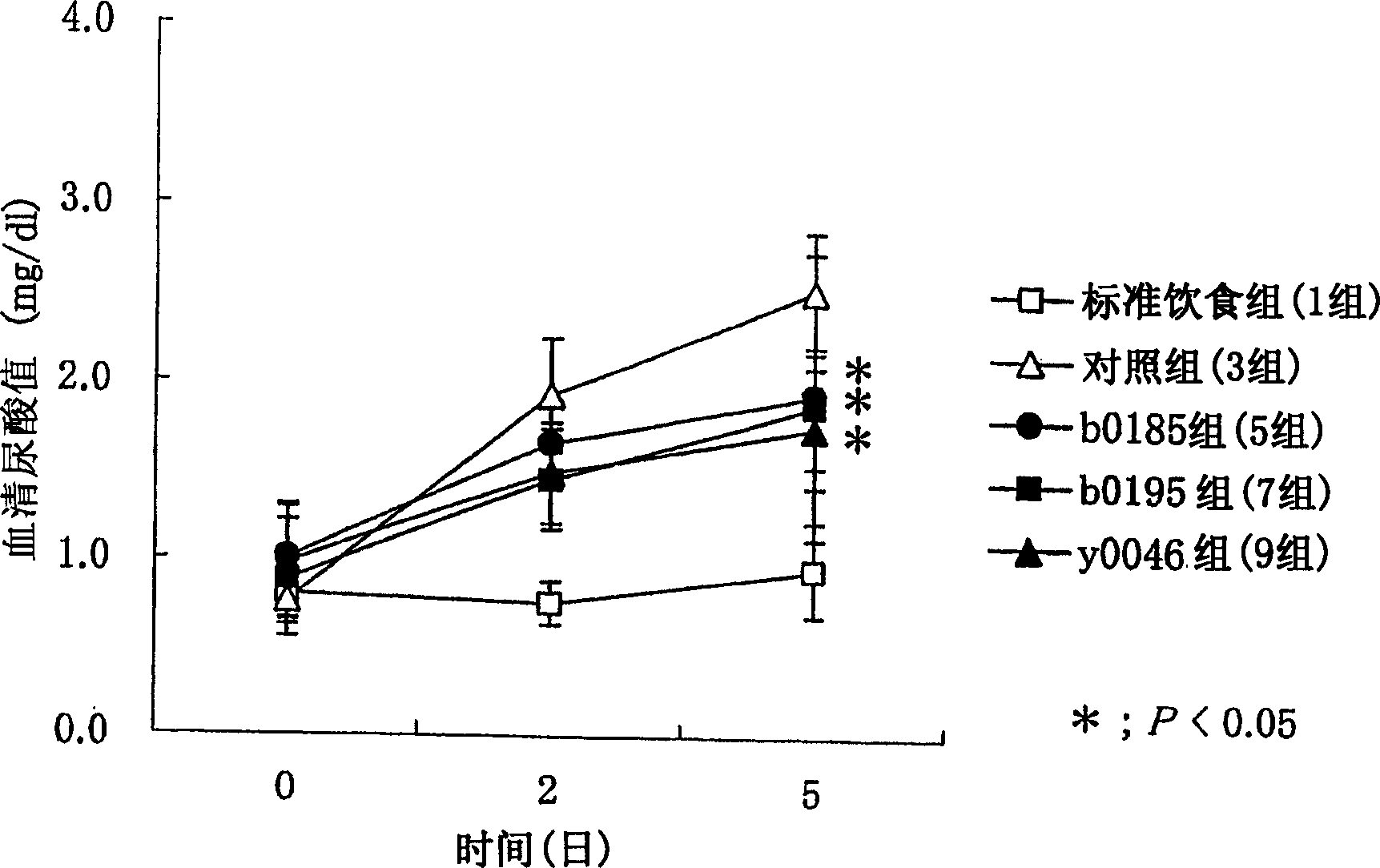
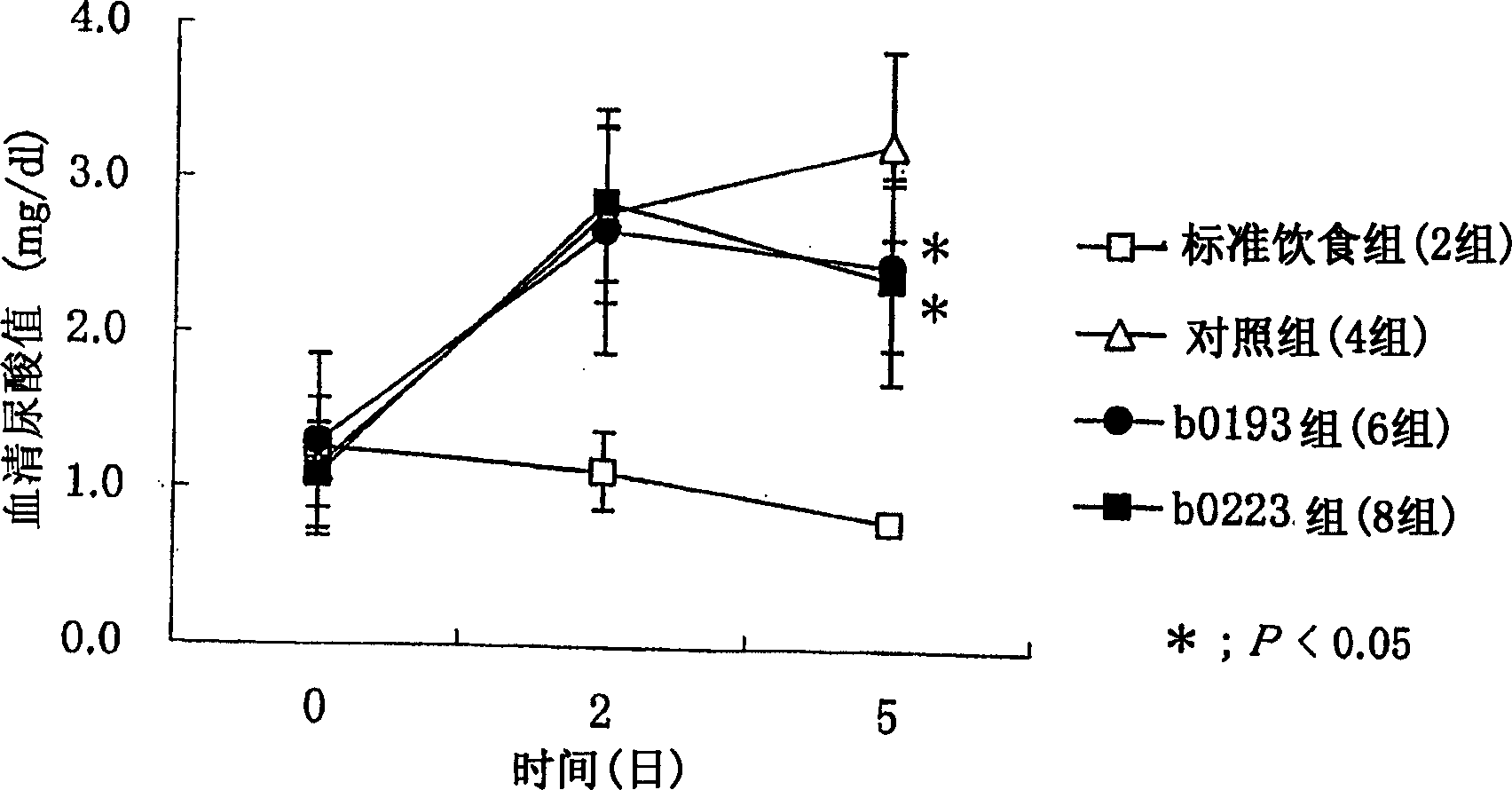
[0399] In this example, food-induced hyperuricemia model animals were produced according to the method described in Clinical Toxicology 13(1), 47-74(1978), and the microorganisms of the present invention were studied according to the method described below on the model Effect of serum uric acid levels in animals.
[0400] The microorganisms ONRIC b0185 (FERM BP-10004), ONRICb0193 (FERM BP-10005), ONRIC b0195 (FERM BP-10006), ONRICb0223 (FERM BP-10007) and ONRIC y0046 (FERM BP-10008) of the present invention were used.
[0401] 1. Experimental animals
[0402] Six-week-old Wister rats (5 rats per group) were used.
[0403] 2. Feeding conditions
[0404] After the arrival of the animals, the animals were acclimated for a period of 1 week. During acclimatization, animals were fed MF solid food (produced by Oriental Yeast Co., Ltd.) and provided with drinking water ad libitum. Each animal was housed individually in a stainless steel cage. The light-dark cycle included a light...
preparation example
[0423] In this example, formulation examples of the composition of the present invention are as follows.
[0424] (1) Preparation of fermented soybean milk
[0425] Beverage forms of the compositions of the present invention are prepared by weighing and mixing the components separately as shown in the formulation below.
[0426] Lactobacillus ONRIC b0185-Fermented Soymilk 100ml
[0427] Lactoligosaccharides (55% content) 10.0g
[0428] Vitamins and minerals in moderation
[0429] Appropriate amount of seasoning
[0430] Appropriate amount of water
[0431] 150ml total
[0432] by adding 10 8 One cell of Lactobacillus ONRIC b0185 (FERM BP-10004) was added to 1 liter of soybean milk (protein content: about 5 g / 100 ml) and fermented at 37° C. for 48 hours to prepare Lactobacillus ONRIC b0185-fermented soybean milk. Its bacterial cell content is about 1×10 9 cells / ml.
[0433](2) Preparation of fermented milk
[0434] The fermented milk form of the composition of the inv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com