Epoxy resin, process for producing the same, epoxy resin composition containing the same, and cured object
A technology of epoxy resin and hydroxide, which is applied in the field of epoxy resin, its manufacture, epoxy resin composition using the epoxy resin and cured products, can solve the problem of low hygroscopicity, unsatisfactory adhesion, low Viscosity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
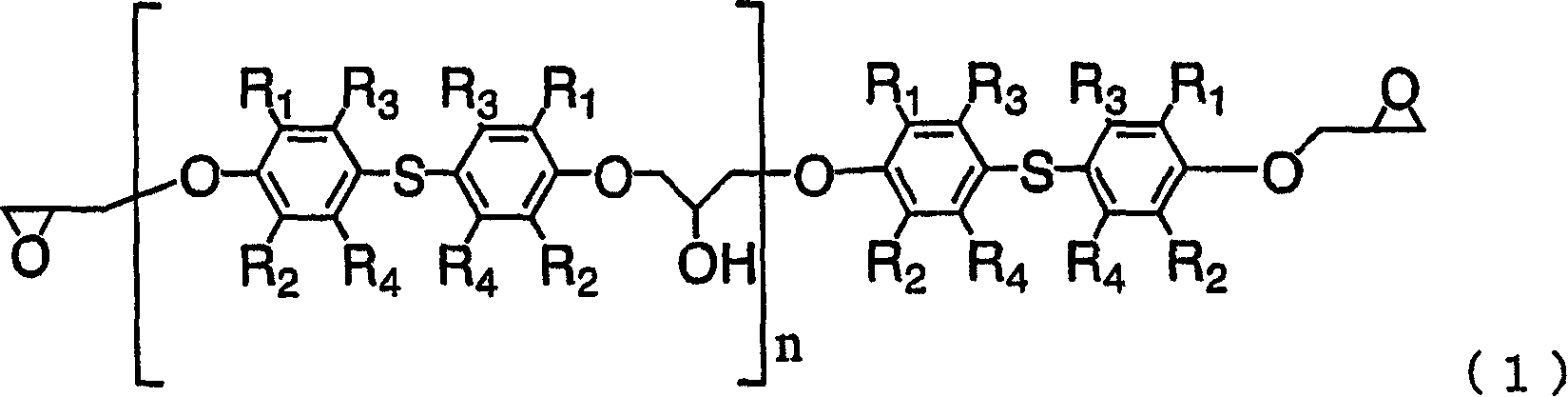
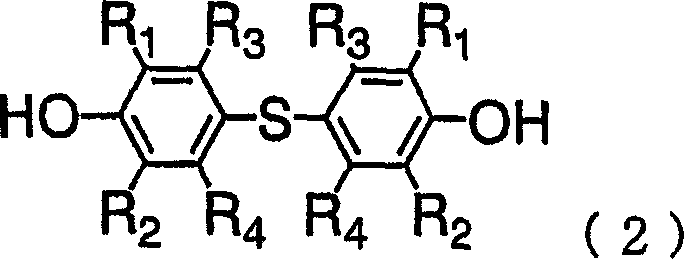
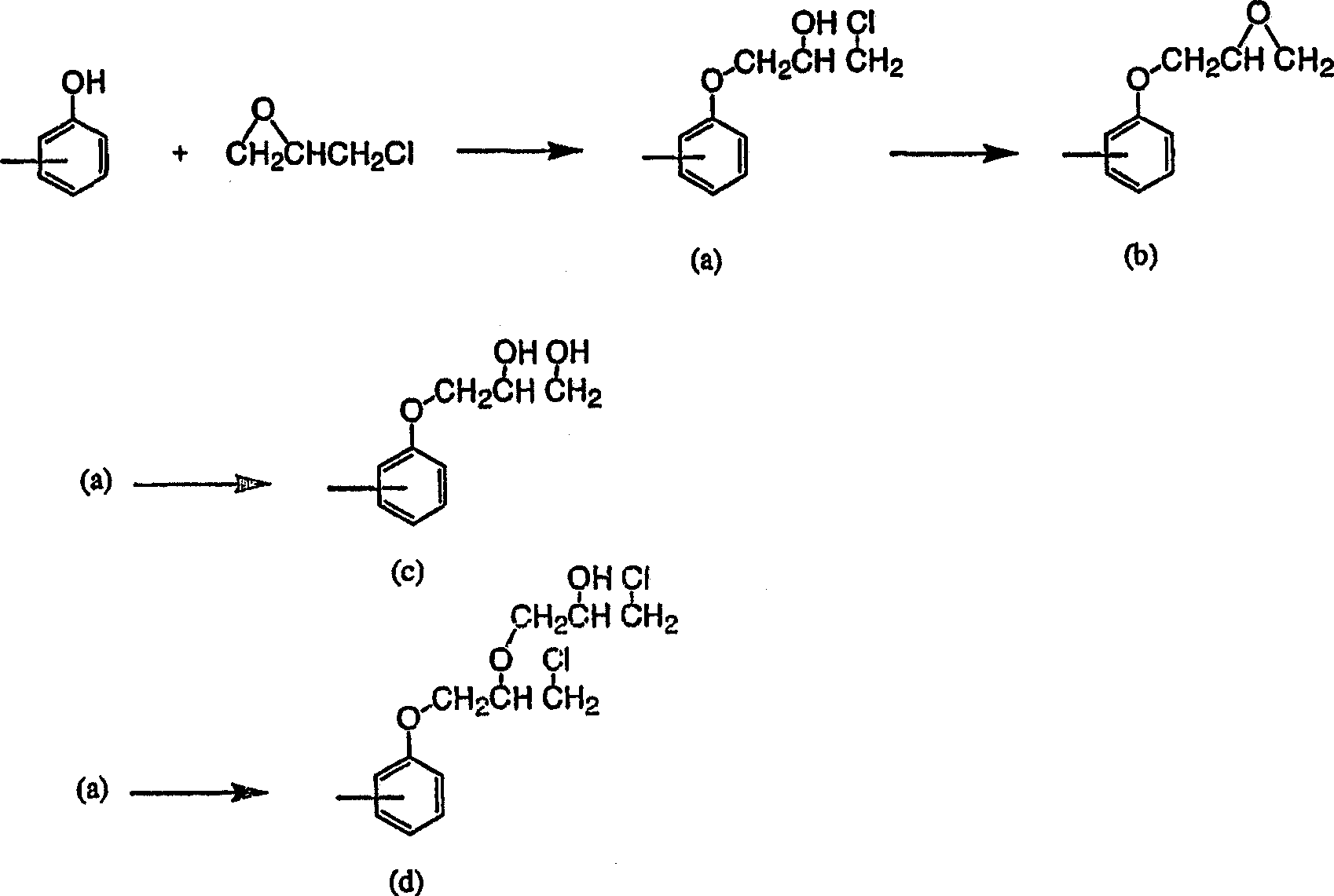
[0048] 240g of DHS was dissolved in 240g of DEGME and 1480g of epichlorohydrin, and reflux was performed under reduced pressure, while 108.4g of 48% sodium hydroxide aqueous solution was added dropwise at 45°C for 4 hours. During this process, generated water was removed from the system by azeotroping with epichlorohydrin, and distilled epichlorohydrin was condensed and returned to the system. After the dropwise addition, the reaction was continued for 1 hour. After that, the generated salt was removed by filtration, and after washing with water, DEGME and epichlorohydrin were distilled off to obtain 302 g of a colorless, transparent, liquid crude epoxy resin. The epoxy equivalent is 248, and the hydrolyzable chlorine is 2100ppm. The purity of DGS (epoxy monomer) in the resin was 91.0 wt%, and the content of the epoxy dimer containing two bisphenol compound units was 5.7 wt%. In addition, the content of the above-mentioned monoepoxide was 3.3 wt%.
[0049] 100 g of the obta...
Embodiment 2
[0052] Methanol was used to recrystallize 100 g of the epoxy resin obtained in Example 1 to obtain 88 g of white crystalline epoxy resin (epoxy resin B). The epoxy equivalent was 236, the hydrolyzable chlorine was 90 ppm, the purity of DGS in the resin was 98.2 wt%, and the content of epoxy dimer was 1.5 wt%. In addition, the content of the monoepoxide was 0.3 wt%. The temperature of the melting point peak in the DSC measurement of the obtained crystal was 122.2°C, the endothermic heat was 77.2 J / g, and the half width of the endothermic peak was 5.6°C.
Embodiment 3
[0054] Using 240g of DHS, 240g of DEGDME, 900g of epichlorohydrin, and 107.0g of 48% sodium hydroxide aqueous solution, it reacted similarly to Example 1, and obtained 298g of liquid crude epoxy resins. The epoxy equivalent is 253, and the hydrolyzable chlorine is 4600ppm. The purity of DGS in the resin is 88.5wt%, and the content of epoxy dimer is 8.4wt%. In addition, the content of the monoepoxide was 3.1 wt%.
[0055] 100 g of the obtained crude epoxy resin was dissolved in 800 g of MIBK, and 10.3 g of a 10% NaOH aqueous solution was added at 80° C., and reacted for 2 hours. After the reaction, filtration and water washing were performed, and MIBK was distilled off to obtain 94 g of a pure yellow liquid epoxy resin. The epoxy equivalent of the obtained epoxy resin was 242, the hydrolyzable chlorine was 240 ppm, the purity of DGS in the resin was 92.6 wt%, and the dimer content of the bisphenol compound was 6.2 wt%. In addition, the content of the monoepoxide was 1.4 wt%....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Heat absorption | aaaaa | aaaaa |
| Softening point | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com