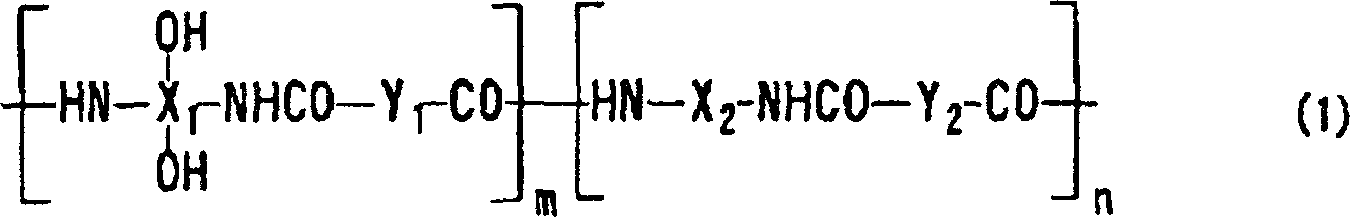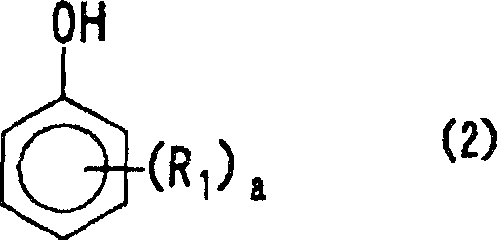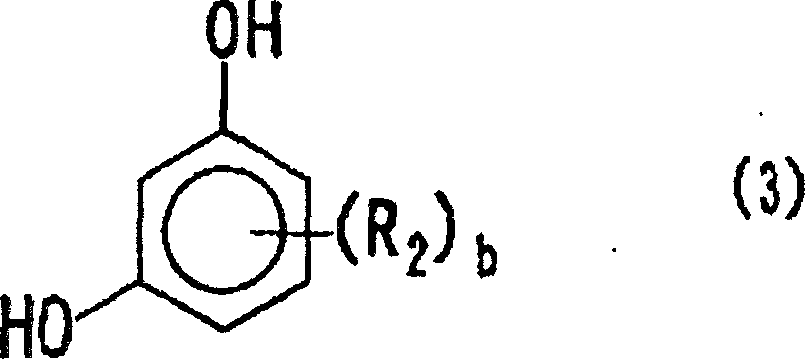Positive photosensitive resin composition
A technology of photosensitive resin and composition, applied in the directions of optics, optomechanical equipment, and photosensitive materials for optomechanical equipment, etc., can solve the problems of insufficient effect, difficulty, change in shape of relief pattern of PBO precursor, etc., Achieves the effect of less change in properties, good shape of hardened relief pattern, and excellent scum removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0086] Reference Example 1
[0087] In a multi-necked flask (separable flask) with a capacity of 3 L, 183.1 g (0.5 moles) of 2,2'-bis(3-amino-4-hydroxyphenyl) hexafluoropropane, 640.9 g of N,N-Dimethylacetamide (DMAC), 63.3 g (0.8 moles) of pyridine, formed a homogeneous solution. 118.0 g (0.4 mol) of 4,4'-diphenyl ether diformyl chloride dissolved in 354 g of diethylene glycol dimethyl ether (DMDG) was added dropwise thereto from a dropping funnel. At this time, the multi-necked flask was cooled in a water bath at 15 to 20°C. The time required for the dropwise addition was 40 minutes, and the temperature of the reaction liquid was at most 30°C.
[0088] After 3 hours of dripping, add 30.8g (0.2 moles) of 1,2-cyclohexyl dicarboxylic anhydride to the reaction solution, leave it for 15 hours with stirring at room temperature, and block all of the polymer chain with carboxycyclohexyl amide groups. 99% of amine end groups. The reaction rate at this time can be easily calculat...
reference example 2
[0090] Reference Example 2
[0091] In a multi-necked flask with a capacity of 2L, mix and stir 197.8g (0.54 mole) 2,2'-bis(3-amino-4-hydroxyphenyl)-hexafluoropropane, 75.9g (0.96 mole) at room temperature (25°C) Pyridine, 692g of DMAC were dissolved. 19.7 g (0.12 mol) of 5-norbornene-2,3- dicarboxylic anhydride was dissolved in 88 g of DMDG, and was added dropwise thereto from a dropping funnel. The time required for the dropwise addition was 40 minutes, and the temperature of the reaction liquid was 28° C. at the maximum.
[0092] After the dripping is over, heat with a hot water bath at 50°C and stir for 18 hours, measure the infrared spectrum of the reaction solution, and confirm that there are 1385 and 1772 cm -1 The characteristic absorption of the imino group.
[0093] Then, it was cooled to 8°C in a water bath, and 142.3 g (0.48 mol) of 4,4'-diphenyl ether dicarboxylic dichloride dissolved in 398 g of DMDG was added dropwise thereto from a dropping funnel. The tim...
Embodiment 1~3 and comparative example 1~2
[0094] Examples 1-3 and Comparative Examples 1-2
[0095] 100 parts by mass of the hydroxyl polyamide (P-1 or P-2) obtained in each of the above-mentioned reference examples, 20 parts by mass of a photosensitive diazoquinone compound having the structure of the following formula Q-1, 10 parts by mass of the compound of the following formula F The phenolic compounds of -1 to F-4 structures were dissolved in 170 parts by mass of GBL, and then filtered through a 0.2 μm filter to prepare the positive photosensitive resins of Examples 1 to 3 and Comparative Examples 1 to 2 described in Table 1. combination.
[0096]
[0097]
[0098] (1) Evaluation of composition characteristics
[0099] The above-mentioned positive photosensitive resin composition was spin-coated on a 5-inch silicon wafer with a spin coater (Dspin636) manufactured by Dainippon Sculin Manufacturing Co., Ltd., and pre-baked at 130° C. for 180 seconds with a hot plate. A coating film of 10.7 μm was formed. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com