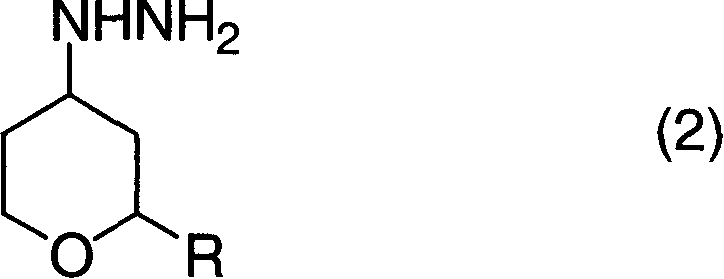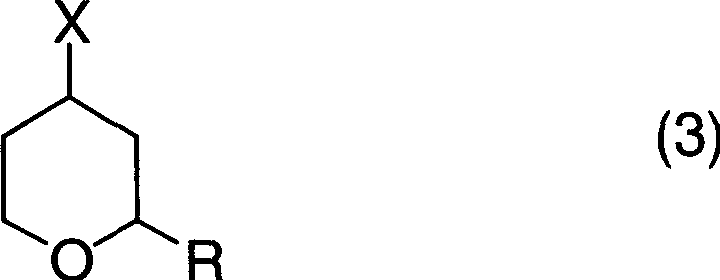Process for production of 4-aminotetrahydropyrans and salts thereof with acids, intermediates for the process, and process for production thereof
A technology of aminotetrahydropyran compound and tetrahydropyran compound, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of complex reaction operation, complex reaction system, and low yield of target products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] (1) Synthesis of 4-hydrazinotetrahydropyran
[0073] In a glass flask with a stirring device, a thermometer and a reflux condenser with an inner volume of 500ml, add 134.7g (710mmol) of tetrahydropyran-4-methanesulfonate with a purity of 95%, 256ml (5.27mol) of Hydrazine monohydrate (hydrazine monohydrate) and 256 ml of ethanol were reacted at 70-80° C. for 3 hours under a nitrogen atmosphere while stirring. After the reaction, the reaction liquid was cooled to room temperature, and after adding 98 ml (784 mmol) of 8 mol / l sodium hydroxide aqueous solution, it was concentrated under reduced pressure. After adding 500 ml of toluene to the concentrate, it was filtered, and the filtrate was again concentrated under reduced pressure. The precipitated solid was filtered off to obtain 45.0 g of 4-hydrazinotetrahydropyran with a purity of 93% (area percentage according to gas chromatography) (isolation yield: 51%) as a yellow liquid.
[0074] The physical property values o...
Embodiment 2
[0080] (1) Synthesis of 4-hydrazinotetrahydropyran hydrochloride
[0081] In a glass flask with a stirring device, a thermometer and a reflux condenser with an inner volume of 200ml, add 10.0g (50mmol) of tetrahydropyran-4-methanesulfonate with a purity of 95%, 26ml (530mmol) of hydrazine monohydrate The material and 26ml of ethanol were reacted at 75° C. for 3 hours under a nitrogen atmosphere while stirring. After completion of the reaction, the reaction liquid was cooled to room temperature, and after adding 14 g (72.6 mmol) of 28% by weight sodium methoxide methanol solution, it was concentrated under reduced pressure. After adding 50 ml of toluene to the concentrate, it was filtered, and the filtrate was again concentrated under reduced pressure. The concentrate was cooled to 0° C., added with 50 ml of methanol and 6.5 ml (78 mmol) of 12 mol / l hydrochloric acid, and then concentrated under reduced pressure. The concentrate was recrystallized using ethanol and toluene to...
Embodiment 3
[0091] Synthesis of 4-aminotetrahydropyran hydrochloride
[0092] In a glass flask with a stirring device, a thermometer and a reflux condenser with an inner volume of 50ml, add 1.0g (6.55mmol) of 4-hydrazinotetrahydro with a purity of 99% synthesized in the same manner as in Example 2 (2) above Pyran, 200 mg of developed Raney nickel and 5 ml of ethanol were reacted in an argon atmosphere at 75° C. for 20 hours. After the reaction, the reaction solution was cooled to room temperature, the reaction solution was filtered, and the filtrate was analyzed by gas chromatography (internal standard method) to find that 246 mg of 4-aminotetrahydropyran was generated (reaction yield: 37%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com