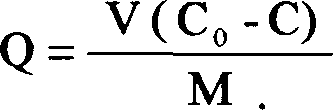Method for synthesizing dithioamino formic acid and diivinyl triamineethyl polymers
A technology of diethylenetriamineethyl dithiocarbamate and a synthesis method, which is applied in the field of synthesis of dithiocarbamate polymers, can solve problems such as unfavorable popularization and application, high reaction temperature, and long suction filtration time, and achieve Conducive to popularization and application, simple operation, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
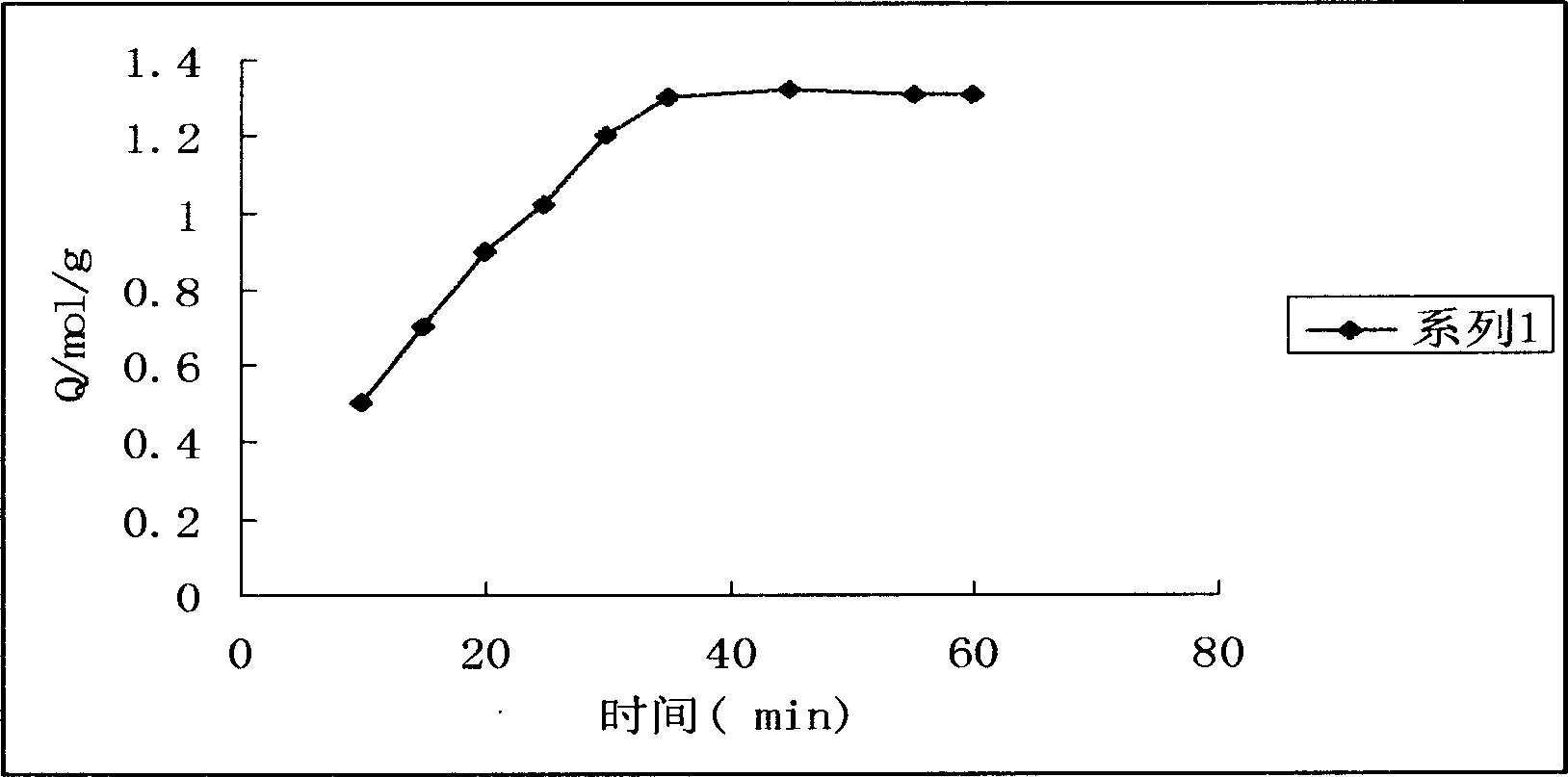
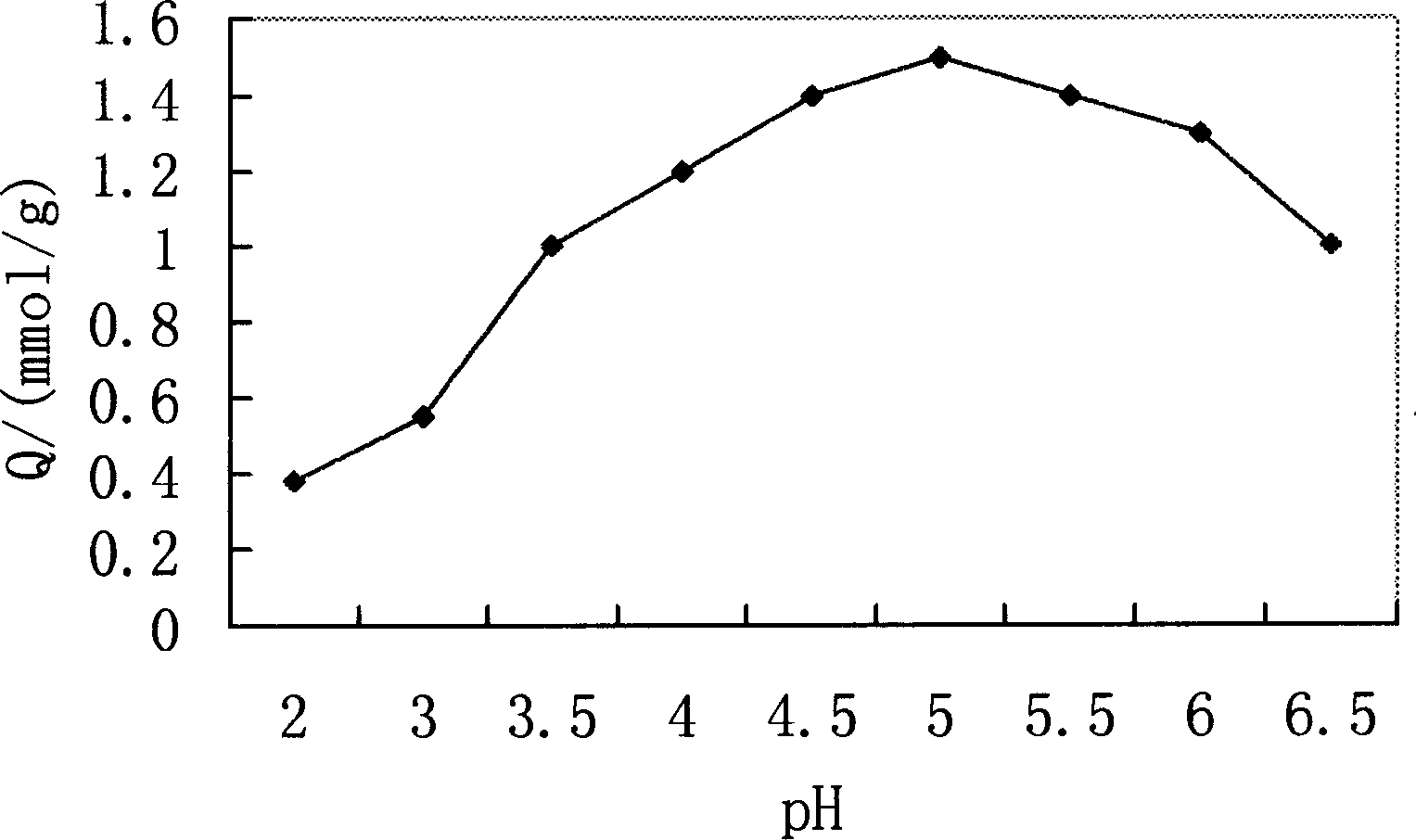
Image
Examples
Embodiment 1
[0024] A kind of operation sequence of the synthetic method of diethylenetriamine ethyl dithiocarbamic acid polymer is as follows:
[0025] Under normal pressure, in a three-necked round-bottomed flask equipped with electromagnetic stirring and reflux condenser, the following operations were performed in sequence:
[0026] (1), sequentially add medicine and stir
[0027] ① first add water 100ml and sodium hydroxide 12g and stir;
[0028] ②Finally, add 10.75ml of diethylenetriamine and stir;
[0029] ③ Slowly add 12.06ml of carbon disulfide dropwise and stir at a rate of 0.5 drops per second.
[0030] (2), react
[0031] After the carbon disulfide in steps ①~③ was added dropwise, the reaction was carried out at room temperature for 1.5 hours to obtain an orange-yellow reaction liquid.
[0032] (3) Stir again and carry out polymerization reaction
[0033] In the orange-yellow liquid obtained in step (2), slowly add 7.88ml of 1,2-dichloroethane dropwise and stir at a rate of...
Embodiment 2
[0048] A kind of operation sequence of the synthetic method of diethylenetriamine ethyl dithiocarbamic acid polymer is as follows:
[0049] Under normal pressure, in a three-necked round-bottomed flask equipped with electromagnetic stirring and reflux condenser, the following operations were performed in sequence:
[0050] (1), sequentially add medicine and stir
[0051] ① first add water 100ml and sodium hydroxide 14g and stir;
[0052] ②Finally, add 10.75ml of diethylenetriamine and stir;
[0053] ③ Slowly add 11.09ml of carbon disulfide dropwise and stir at a rate of 1 drop per second.
[0054] (2), react
[0055] After the carbon disulfide in steps ①~③ was added dropwise, the reaction was carried out at room temperature for 1 hour to obtain an orange-yellow reaction liquid.
[0056] (3) Stir again and carry out polymerization reaction
[0057] In the orange-yellow liquid obtained in step (2), slowly add 7.96ml of 1,2-dichloroethane dropwise and stir at a rate of 1 dro...
Embodiment 3
[0066] A kind of operation sequence of the synthetic method of diethylenetriamine ethyl dithiocarbamic acid polymer is as follows:
[0067] Under normal pressure, in a three-necked round-bottomed flask equipped with electromagnetic stirring and reflux condenser, the following operations were performed in sequence:
[0068] (1), sequentially add medicine and stir
[0069] ① first add water 100ml and sodium hydroxide 15g and stir;
[0070] ②Finally, add 11ml of diethylenetriamine and stir;
[0071] ③ Slowly add 12.30ml of carbon disulfide dropwise and stir at a rate of 0.5 drops per second.
[0072] (2), react
[0073] After the carbon disulfide in steps ①~③ was added dropwise, the reaction was carried out at room temperature for 80 minutes to obtain an orange-yellow reaction liquid.
[0074] (3) Stir again and carry out polymerization reaction
[0075] In the orange-yellow liquid obtained in step (2), slowly add 7.72ml of 1,2-dichloroethane dropwise and stir at a rate of 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com