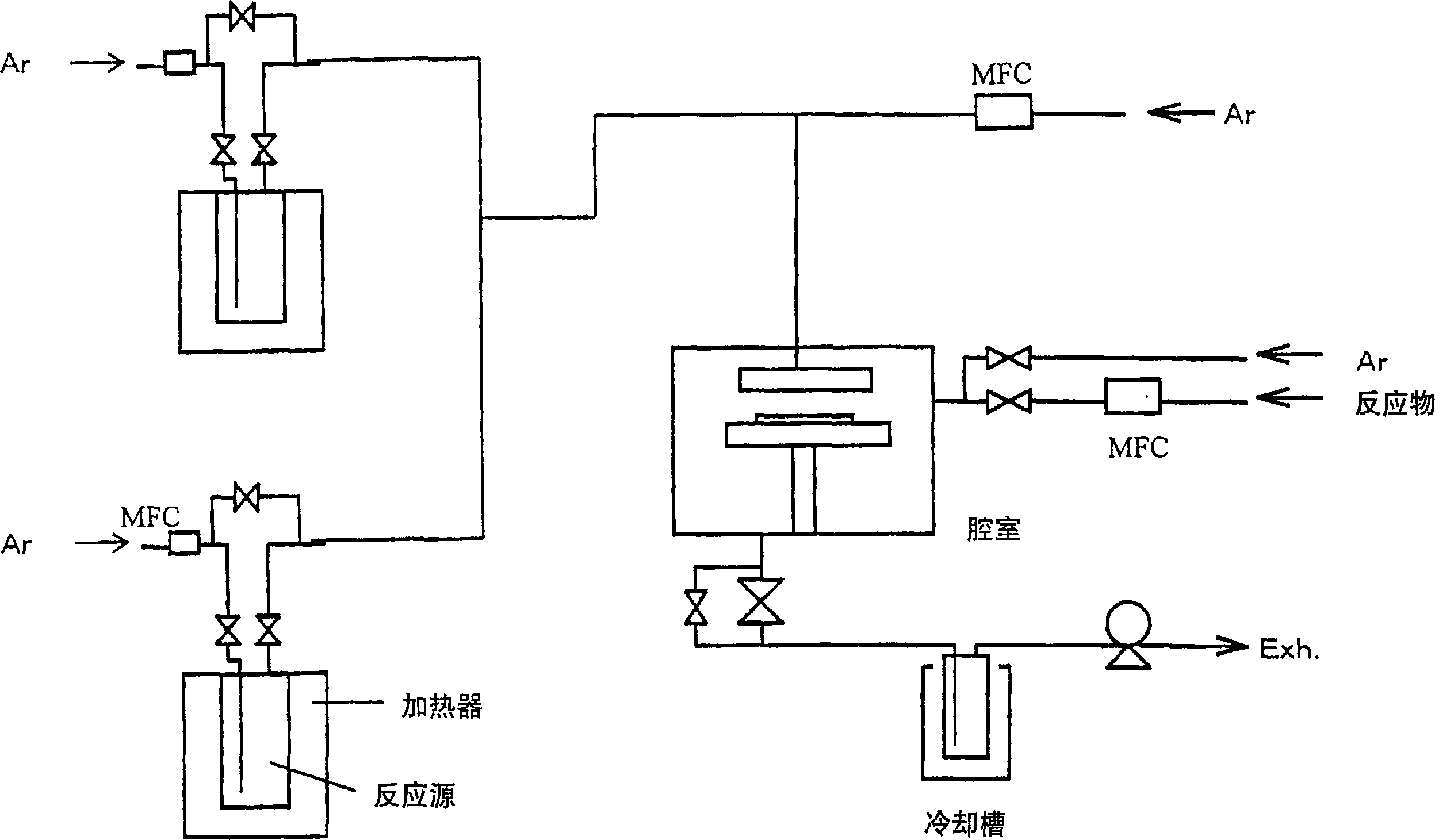
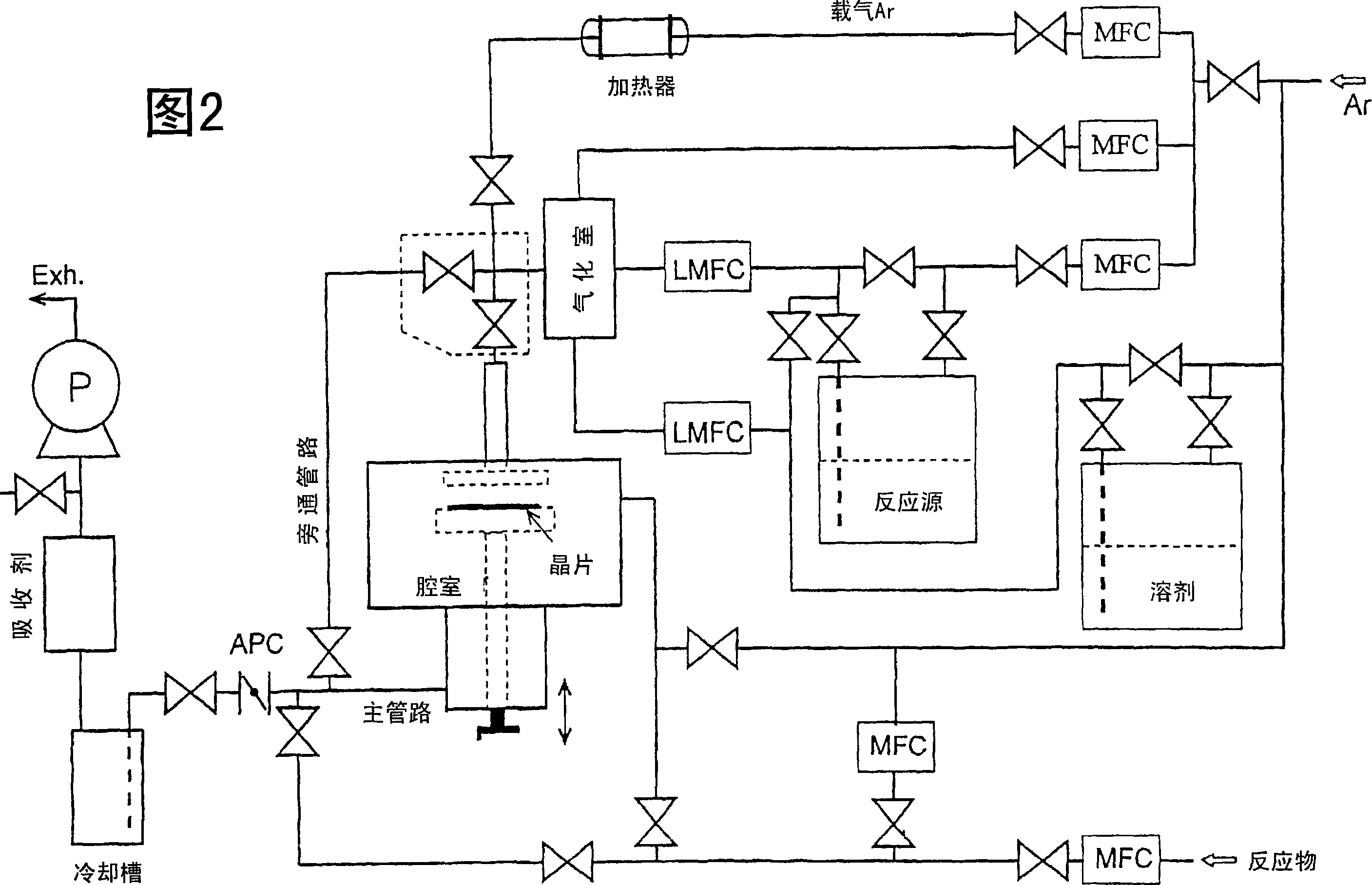
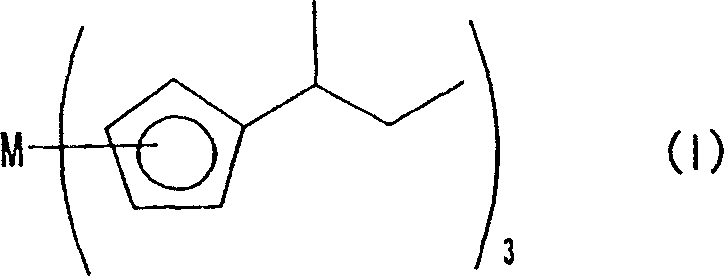
Rare earth metal complex, material for thin-film formation, and process for producing thin film
A technology of complexes and metal atoms, applied in rare earth metal compounds, chemical instruments and methods, metal material coating technology, etc., can solve problems such as low volatility and increased molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] [Example 1] Production of tris(sec-butylcyclopentadiene)yttrium
[0043] In a reaction flask replaced with dry argon, 0.948 mol of sodium hydride thoroughly washed with hexane and 30 ml of tetrahydrofuran were added, and 0.948 mol of sec-butylcyclopentadiene (racemate) was added dropwise at a temperature of 10°C or below . After the dropwise addition, it was stirred at 30°C until no gas was generated and a solution was obtained. The solution was cooled to 10°C or below, and while adding it to 0.190 mol of yttrium trichloride and 30 ml of tetrahydrofuran, the temperature of the reaction system was cooled to 10°C or below. After stirring at room temperature for 10 hours, the solvent was distilled off to obtain a residue. 600 ml of toluene was added to the residue, and the solid phase was filtered. After removing the solvent from the obtained filtrate by vacuum distillation, a light-shielding distillation apparatus was used to perform vacuum distillation. 39.4 g (yield 46%) of...
Embodiment 2
[0053] [Example 2] Production of tris(sec-butylcyclopentadiene)lanthanum
[0054] In a reaction flask replaced with dry argon, 0.816 mol of sodium hydride thoroughly washed with hexane and 30 ml of tetrahydrofuran were added, and 0.816 mol of sec-butylcyclopentadiene (racemate) was added dropwise at a temperature of 10°C or below . After the dropwise addition, it was stirred at 30°C until no gas was generated and a solution was obtained. The solution was cooled to 10°C or lower, and while adding it to 0.204 mol of lanthanum trichloride and 30 ml of tetrahydrofuran, the temperature of the reaction system was cooled to 10°C or lower. After stirring for 30 minutes at room temperature, the solid phase was filtered. After removing the solvent from the obtained filtrate by vacuum distillation, a light-shielding distillation apparatus was used to perform vacuum distillation. 43.1 g (yield 42%) of light yellow liquid was prepared from the fraction with 100 Pa and distilled vapor temperatu...
Embodiment 3
[0064] [Example 3] Production of Tris(sec-butylcyclopentadiene)praseodymium
[0065] In a reaction flask replaced with dry argon, 0.158 mol of sodium hydride thoroughly washed with hexane and 50 ml of tetrahydrofuran were added, and 0.158 mol of sec-butylcyclopentadiene (racemate) was added dropwise at a temperature of 10°C or below . After the dropwise addition, it was stirred at 30°C until no gas was generated and a solution was obtained. The solution was cooled to 10°C or below, and while adding it to 0.040 mol of praseodymium trichloride and 15 ml of tetrahydrofuran, the temperature of the reaction system was cooled to 10°C or below. After stirring at room temperature for 10 hours, the solvent was distilled off to obtain a residue. 100 ml of hexane was added to the residue, and the solid phase was filtered. After removing the solvent from the obtained filtrate by vacuum distillation, a light-shielding distillation apparatus was used to perform vacuum distillation. 11.3 g (yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com