Chiral drug intercalation hydrotalcite and its preparing method
A technology of chiral drugs and hydrotalcites, which is applied in the direction of pharmaceutical formulations, drug combinations, organic active ingredients, etc., to achieve effective storage effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
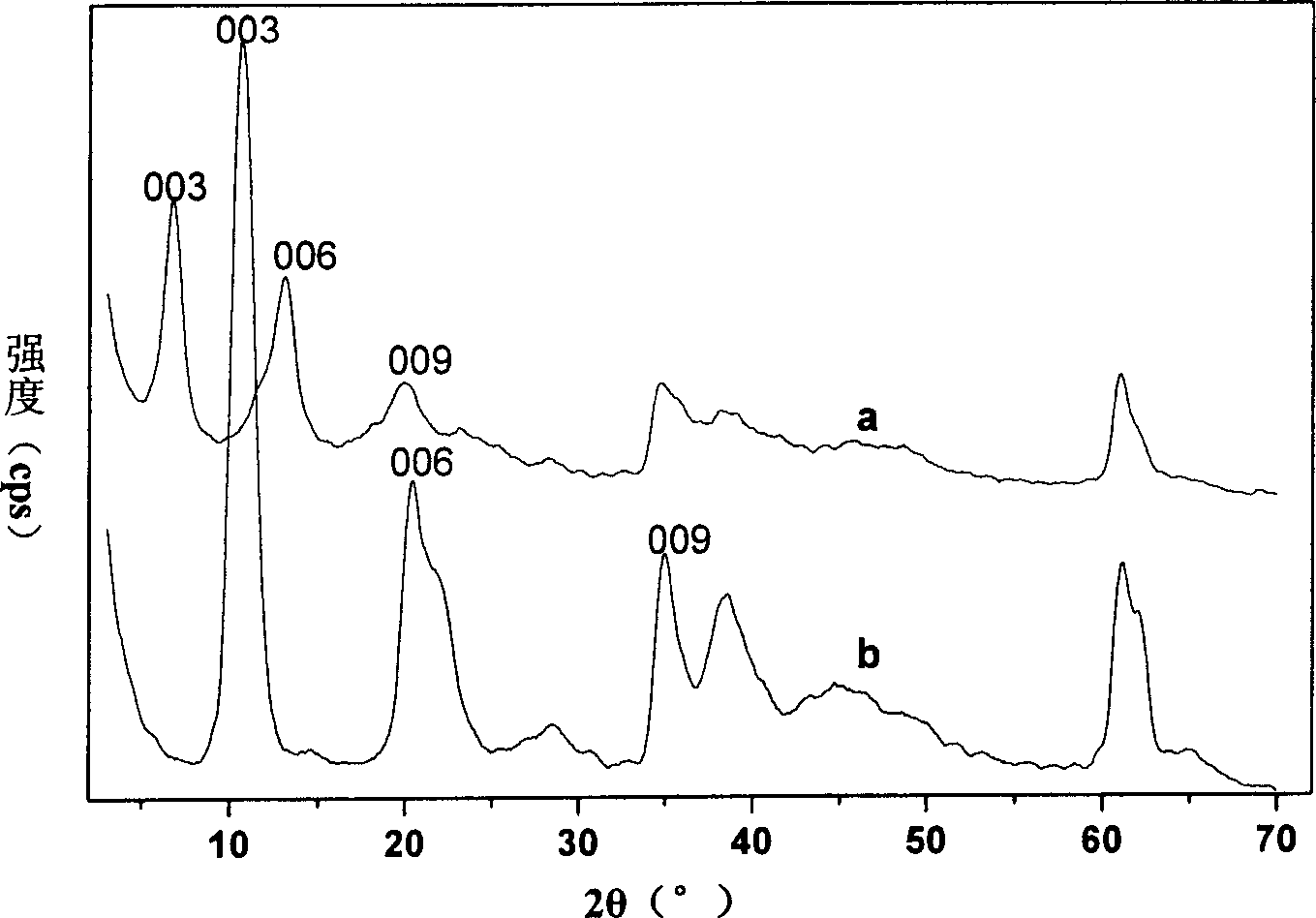
[0024] Step A: take by weighing 15.36g Mg(NO 3 ) 2 .6H 2 O and 11.25g Al(NO 3 ) 3 .9H 2 O dissolved in 150ml to remove CO 2 , deionized water to prepare a mixed salt solution, and another 7.2g NaOH was dissolved in 200ml to remove CO 2 , deionized water and placed in a 500mL four-necked flask; using the single-drop co-precipitation method, in a 70°C water bath, N 2 Slowly add the salt solution of the former to the alkali solution of the latter under protection and strong stirring conditions, adjust the pH value to 10.0, and react for 40 hours; wash and filter the product until pH3 -Mg 2 Al-LDHs, its Mg 2+ / Al 3+ =2.
[0025] Step B: According to the molar ratio of chiral drug levodopa / hydrotalcite precursor = 3, first weigh 0.6g (about 3mmol) Levodopa and dissolve it in 400mL to remove CO 2 , deionized water and placed in a 500mL four-necked flask; then weigh 3.6g (about 1 mmol) of the filter cake made by step A and add it, shielded from light, N 2 Protect, vigorous...
Embodiment 2
[0029] Step A: Take a method similar to step A in Example 1 to prepare NO 3 -Mg 2 Al-LDHs precursor.
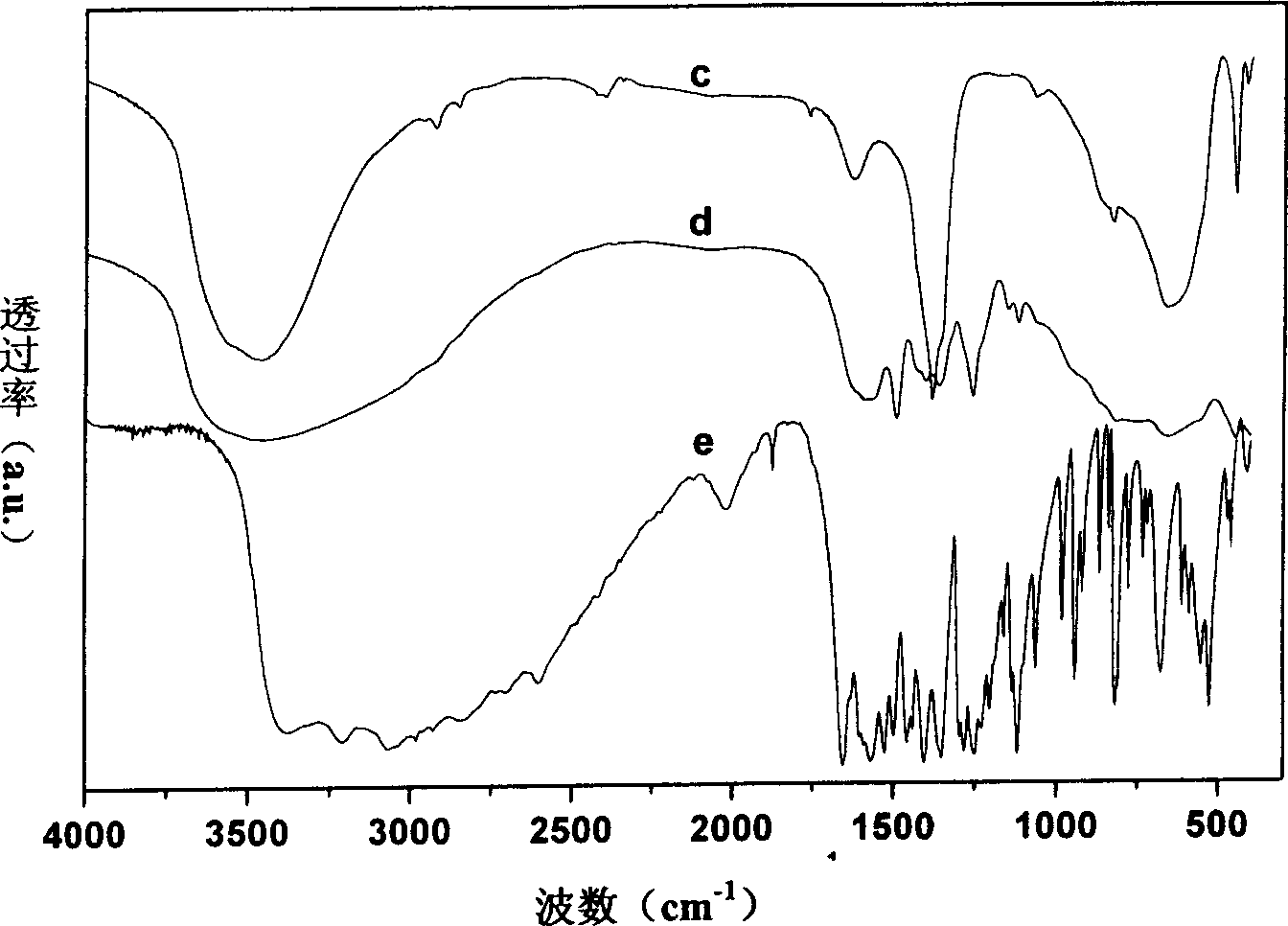
[0030] Step B: According to the molar ratio of chiral drug levodopa / hydrotalcite precursor = 2, first weigh 0.8g (about 4mmol) Levodopa and dissolve it in 400mL to remove CO 2 , deionized water and placed in a 500mL four-neck flask; then weigh 7.0g (about 2mmol) of the filter cake made by step A and add it, shielded from light, N 2 Protect, vigorously stir, adjust the pH to 7.8, ion exchange at room temperature for 2 days; the product is used to remove CO 2 1. Washing and centrifuging with deionized water for 5 times, drying the precipitate at room temperature in vacuum, and then obtaining a layered composite material containing levodopa anion between layers.
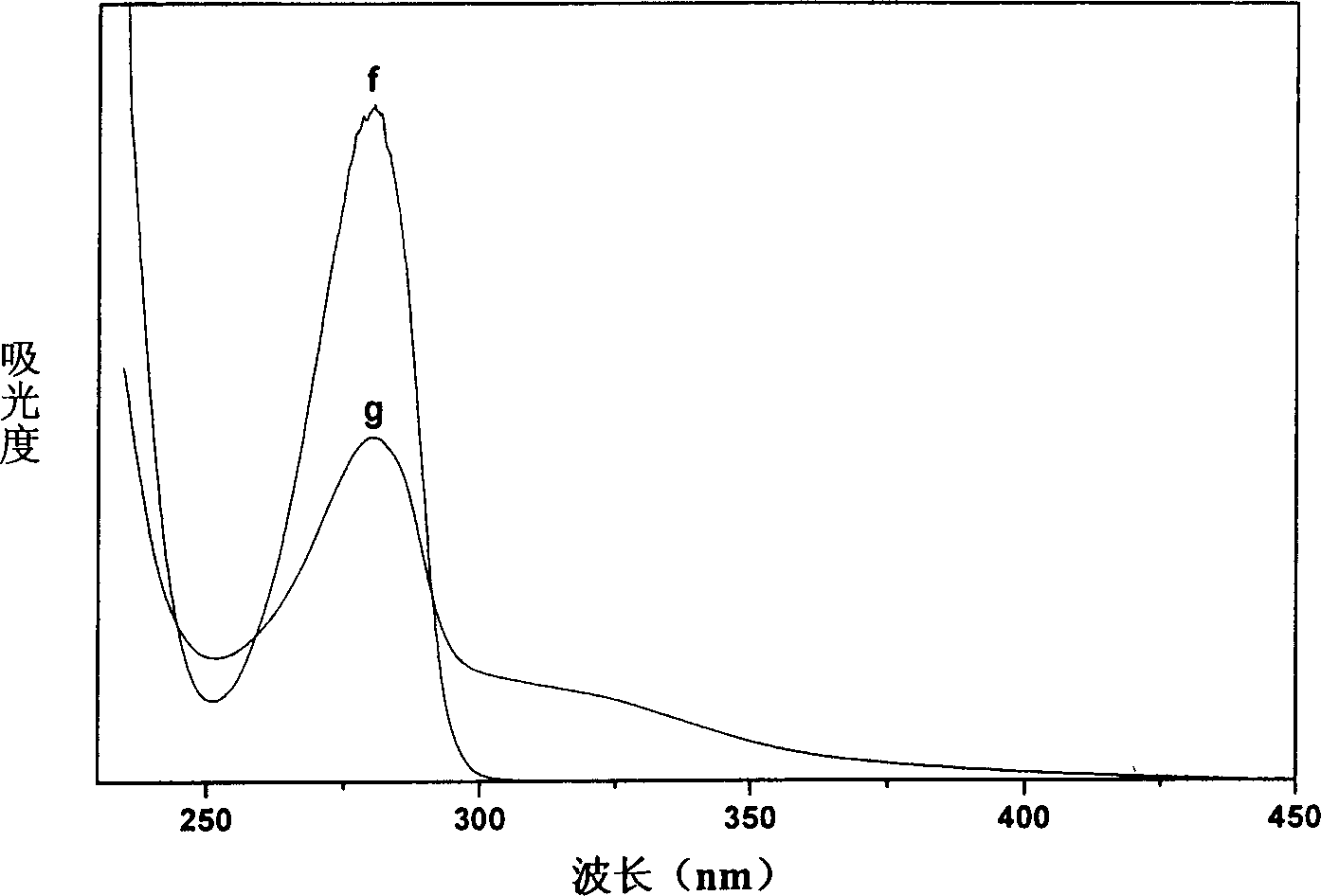
[0031] Step C: Carry out in vitro simulated release test by adopting a method similar to Step C in Example 1, so as to obtain the release curve.
[0032] Elemental analysis can give the chemical formula Mg 4.64 al...
Embodiment 3
[0034] Step A: Take a method similar to step A in Example 1 to prepare NO 3 -Mg 2 Al-LDHs precursor.
[0035] Step B: According to the method similar to Step B in Example 2, after the first ion exchange reaction 2d, the product uses CO 2 , washed with deionized water, and centrifuged for 5 times to obtain a precipitated product.
[0036] Step C: Weigh 0.5g (about 2.5mmol) Levodopa dissolved in 400mL to remove CO 2 , deionized water and placed in a 500mL four-necked flask; 2 Under the conditions of protection and strong stirring, add the precipitate obtained from the above step B to the system, adjust the pH to 7.8, and perform the second ion exchange at room temperature for 2 days; the product is used to remove CO 2 1. Washing and centrifuging with deionized water for 5 times, drying the precipitate at room temperature in vacuum, and then obtaining a layered composite material containing levodopa anion between layers.
[0037] Step D: Take the method similar to Step C in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com