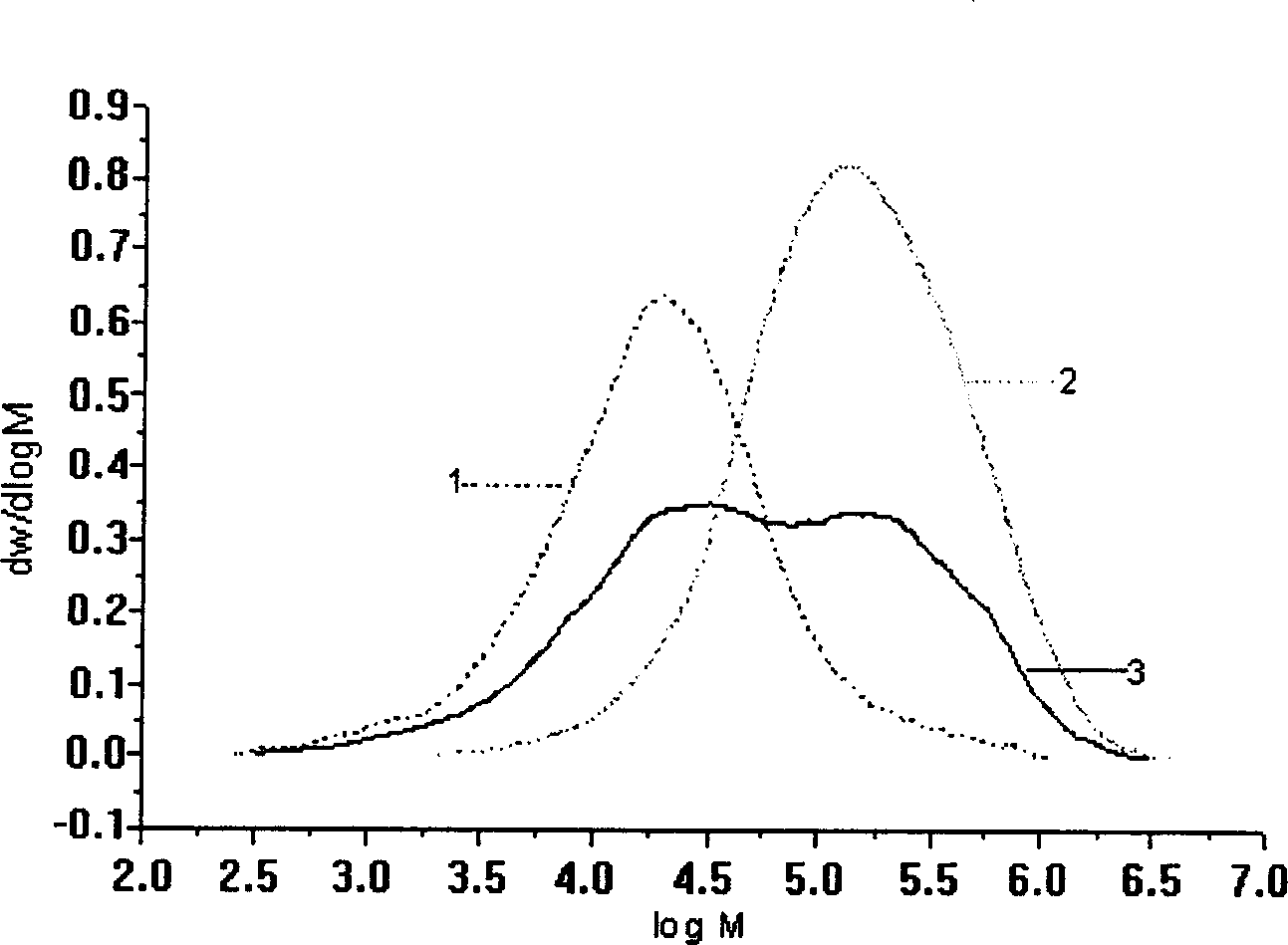
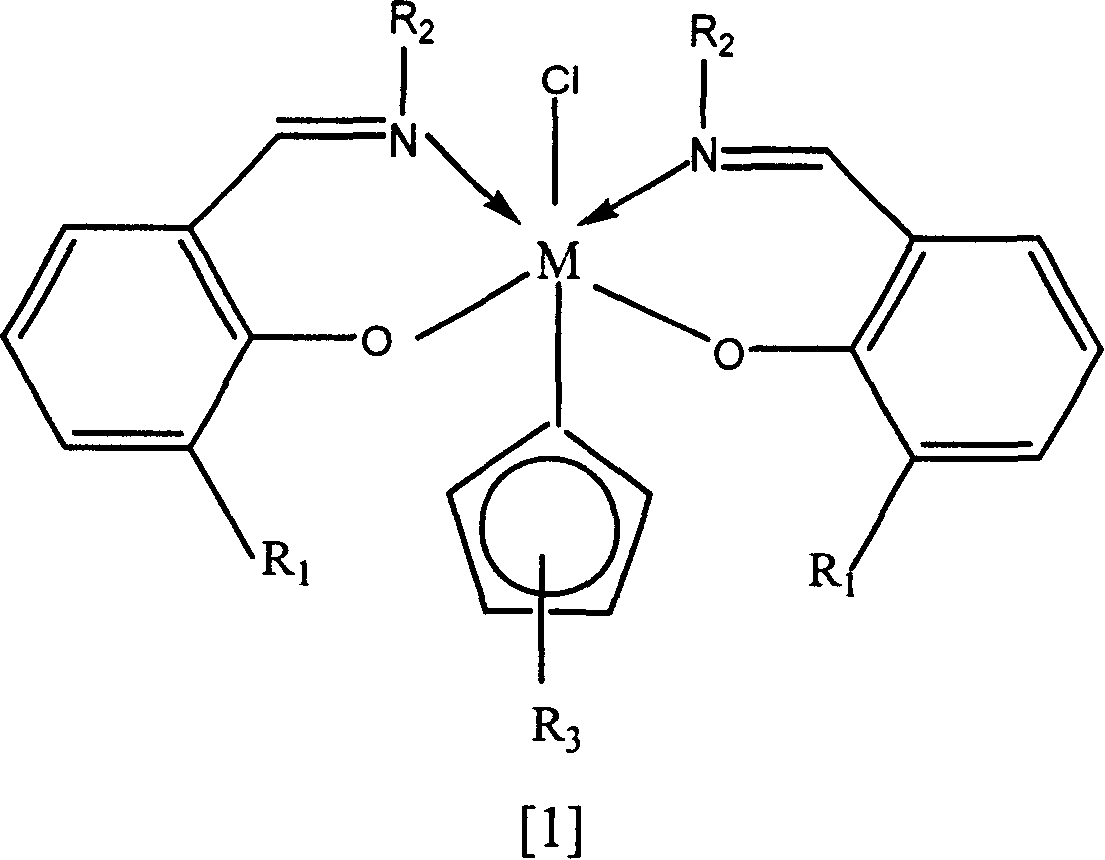
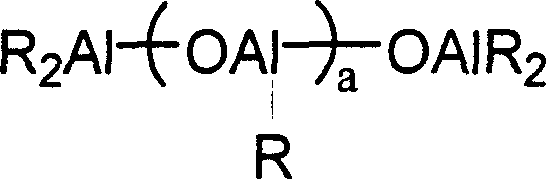Composite catalytic system for preparing wide/dual-peak distributed high density polyethylene
A high-density polyethylene and bimodal distribution technology is applied in the field of composite catalytic systems for preparing broad/bimodal high-density polyethylene, which can solve the problems of low branching degree, inability to achieve balanced compatibility of processability and strength, and the like. To achieve the effect of improving processing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Synthesis of compound (A):
[0051] Bis(3-tert-butylsalicylidenecyclohexyl)-(cyclopentadienyl)zirconium chloride.
[0052] (1) Preparation of ligand compound 3-tert-butyl salicylidene cyclohexylamine:
[0053] Take 17.8g (0.1mol) of 3-tert-butylsalicylaldehyde and 0.2mol of cyclohexylamine and add it to 100ml of ethanol medium, heat to reflux under stirring, react for 2 hours, cool to room temperature, and a large number of crystals are produced. Filtration and recrystallization of the solid with 30 ml of ethanol solvent finally gave the ligand 3-tert-butyl salicylidene aniline with a yield of 92%.
[0054] 1 H NMR (CDCl 3 , 500MHz) δ: 13.93 (br, 1H, D 2 O, OH), 8.64(s, 1H, CH=N), 7.46~6.96(m, 8H, aroma), 1.47(s, 9H, C(CH 3 ) 3 )
[0055] (2) Preparation of the lithium salt of 3-tert-butyl salicylidene cyclohexylamine:
[0056] Add 2.910g (11.5mmol) 3-tert-butylsalicylidenecyclohexylamine and 30ml tetrahydrofuran into a 100ml Schlenk bottle, cool the solution to...
Embodiment 2
[0063] The synthesis of metallocene compound B: (Me 4 Cp) 2 ZrCl 2 Preparation of adducts:
[0064] Under a nitrogen atmosphere, add 20 g (0.164 mol) of newly distilled tetramethylcyclopentadiene to the three-necked flask, add 200 ml of tetrahydrofuran to dissolve, then lower the temperature to below -70°C, and slowly add 65.6 ml (0.164 mol) of n-butyl Lithium-based solution (2.5M), react at this temperature for 1 hour, slowly warm up to room temperature, and react for 4 hours; transfer this solution to a constant pressure dropper, and slowly add it dropwise to the dissolved 19.1g (0.082mol)ZrCl 4 100ml of tetrahydrofuran solution, after dripping, it was gradually raised to room temperature, and then reacted for about 18 hours; after vacuum distillation, evaporated to dryness, dispersed with hexane, filtered, and washed twice with hexane to obtain 39g of purple metallocene Compound B powder, Zr% = 18 (ICP), based on Zr, the yield was 94.1%.
Embodiment 3
[0066] Synthesis of metallocene compound C: (n-BuMeCp) 2 ZrCl 2 Preparation of adducts:
[0067] Under a nitrogen atmosphere, add 20 g (0.147 mol) of newly distilled methyl butyl cyclopentadiene to the three-necked flask, add 200 ml of tetrahydrofuran to dissolve, then lower the temperature to below -70°C, and slowly add 58.9 ml (0.147 mol) of n-butyl Lithium solution (2.5M), react at this temperature for 1 hour, slowly warm up to room temperature, and react for 4 hours; transfer this solution to a constant pressure dropper, and slowly add it dropwise to dissolve 16.43g ( 0.074mol) ZrCl 4 100ml of tetrahydrofuran solution, after dripping, gradually rise to room temperature, and then react for about 18 hours; vacuum distillation, evaporate to dryness, disperse with hexane, filter, wash 2 times with hexane to obtain 35g yellow metallocene Compound C powder, Zr%=17.5 (ICP), based on Zr, the yield was 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com