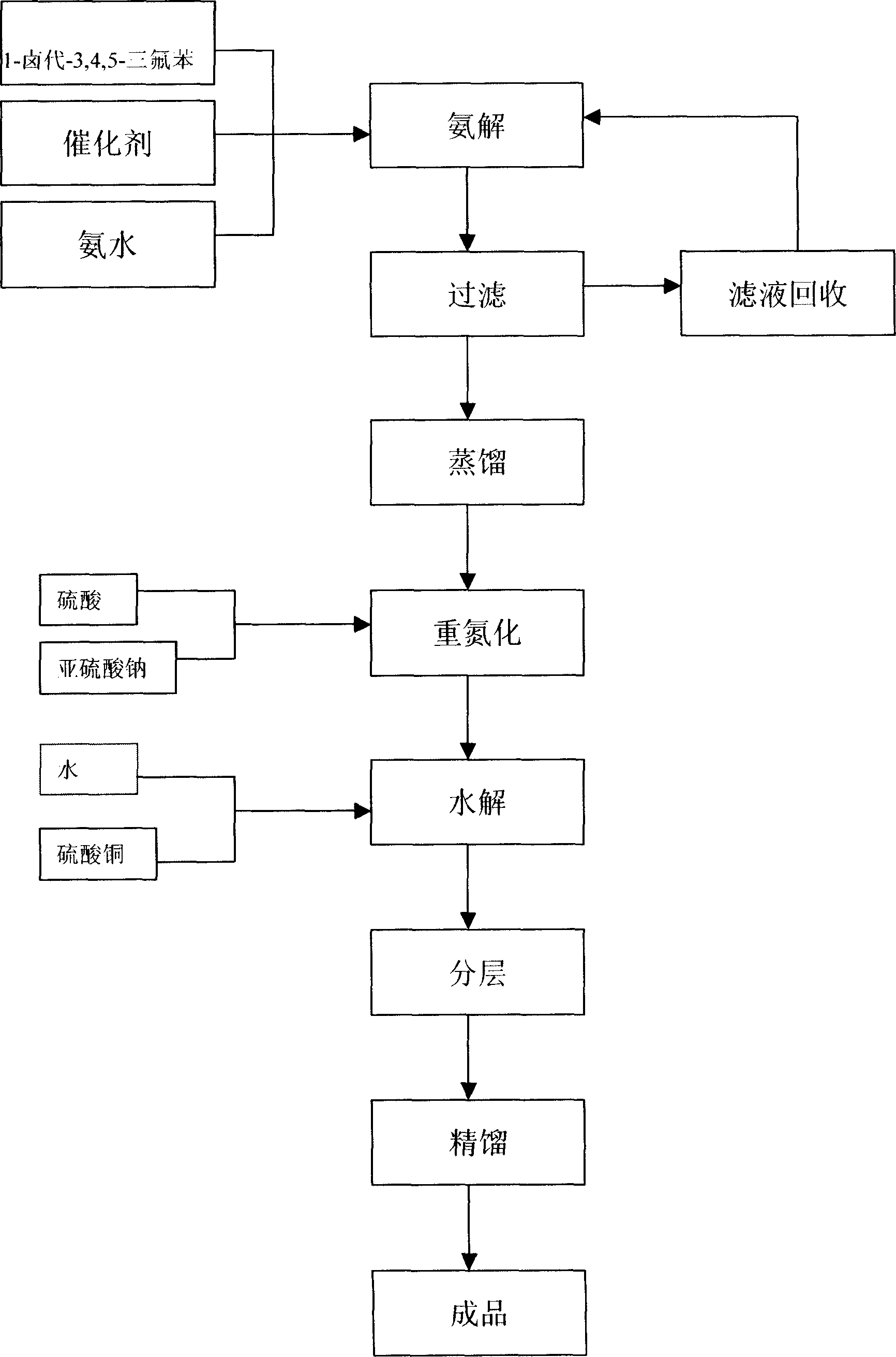
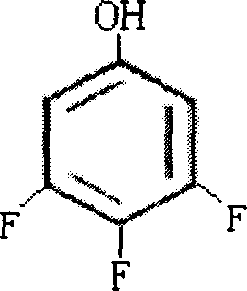
Production tech, of 3,4,5 trifluorophenol
A technology of trifluorophenol and production process, applied in 3 fields of production, can solve the problems of difficult handling, low total reaction yield, harsh Grignard reaction conditions, etc., and achieves the effects of easy availability of raw materials and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] With 1-bromo-3,4,5-trifluorobenzene as the starting material, the following steps are carried out in sequence:
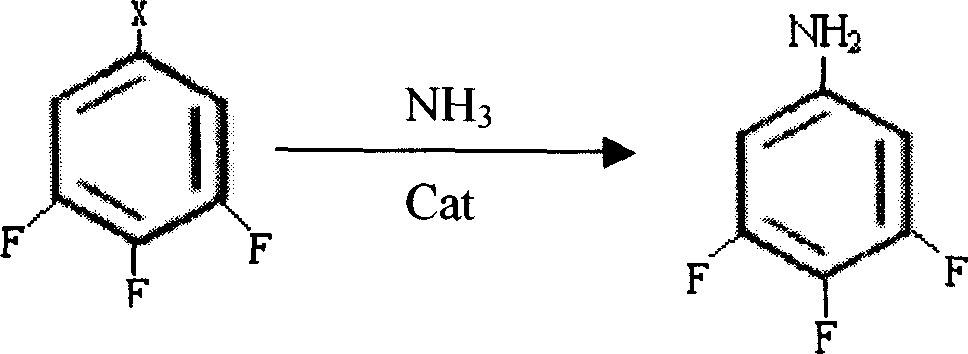
[0021] (1), 6kg of cuprous oxide and 600kg of 1-bromo-3,4,5-trifluorobenzene were added to a 3000L autoclave, and then 2000L of 28% ammonia water was pumped in. Close the autoclave, raise the temperature to 150-160°C with stirring, and the pressure to 3.0 MPa. After 8 hours of reaction, take a sample for analysis. The content of 1-bromo-3,4,5-trifluorobenzene drops to 2 Press into the crystallization pot, stir and cool to 0°C, and filter to obtain 3,4,5-trifluoroaniline product with a yield of 95% and a content of >99.5%. Reaction equation:
[0022]
[0023] (2), add 98% H in 1000L diazotization kettle 2 SO 4 300kg, heat up to 45-50°C, add 75kg of 3,4,5-trifluoroaniline into the kettle at this temperature; after adding, raise the temperature to 65°C and keep it for 25min, cool down to -5°C, add 36kg of nitrous acid dropwise Sodium is dissolved in 120kg...
Embodiment 2
[0028] With 1-bromo-3,4,5-trifluorobenzene as the starting material, the following steps are carried out in sequence:
[0029] (1) 5 g of cuprous sulfate and 600 g of 1-bromo-3,4,5-trifluorobenzene were added to a 3L autoclave, and then 1800 ml of 28% ammonia water was pumped in. Close the autoclave, raise the temperature to 150-160°C with stirring, and the pressure to 3.0 MPa. After 8 hours of reaction, take a sample for analysis. The content of 1-bromo-3,4,5-trifluorobenzene drops to 2 Press into the crystallization pot, stir and cool to 0°C, and filter to obtain 3,4,5-trifluoroaniline product with a yield of 94% and a content of >99.5%.
[0030] (2), add 98% H in 1000ml diazotization kettle 2 SO 4 300g, heat up to 45-50°C, add 75g of 3,4,5-trifluoroaniline into the kettle at this temperature; after adding, raise the temperature to 65°C for 30min, cool down to -5°C, add dropwise 35g of nitrous acid Sodium dissolved in 115g of water, the temperature is -5 ~ 0°C; after drop...
Embodiment 3~11
[0034] In the above table, the amount of catalyst added is per 1 kg of 1-bromo-3,4,5-trifluorobenzene, the amount of catalyst added (kg); the concentration of ammonia water is 28%, and its added amount is per 1 mole of 1-bromo-3 , 4,5-trifluorobenzene, the number of moles added by ammonia water; the amount of 98% sulfuric acid added, the amount of sodium nitrite added, and the amount of copper sulfate added are all per 1 mole of 1-bromo-3,4,5-trifluorobenzene, The number of moles of each of the above reagents added.
[0035] As can be seen from the above table, when the temperature exceeds 170° C., the reaction rate is too fast after the pressure increases, resulting in an increase in by-products. From an economic point of view, 150-160° C. is the preferred step (1). In step (2), the mol ratio of trifluoroaniline to sulfuric acid is about 6, specifically 5.5 to 7, and the mol ratio of trifluoroaniline to sodium nitrite is 0.95 to 1.05. In step (3), trifluoroaniline The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com