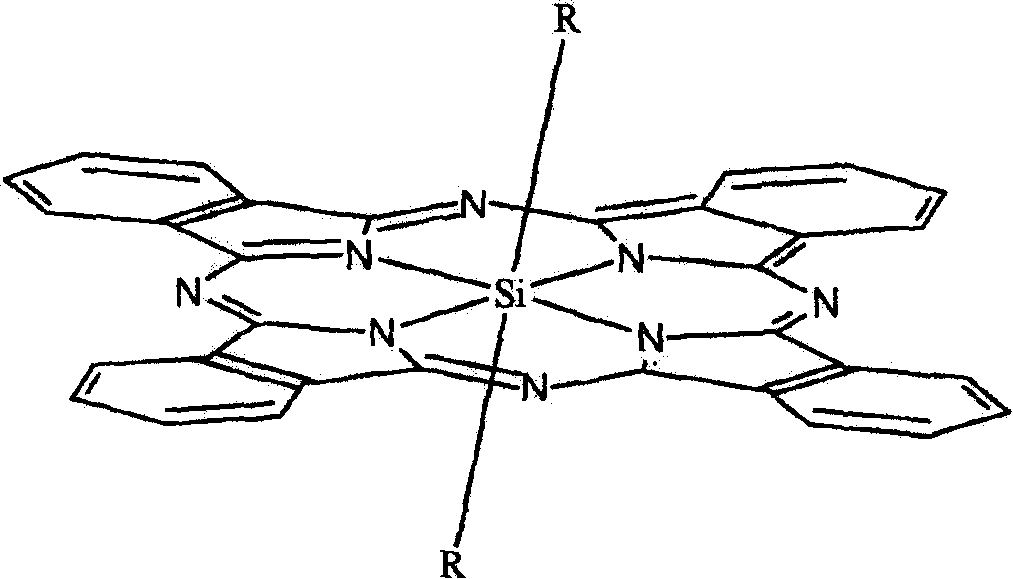
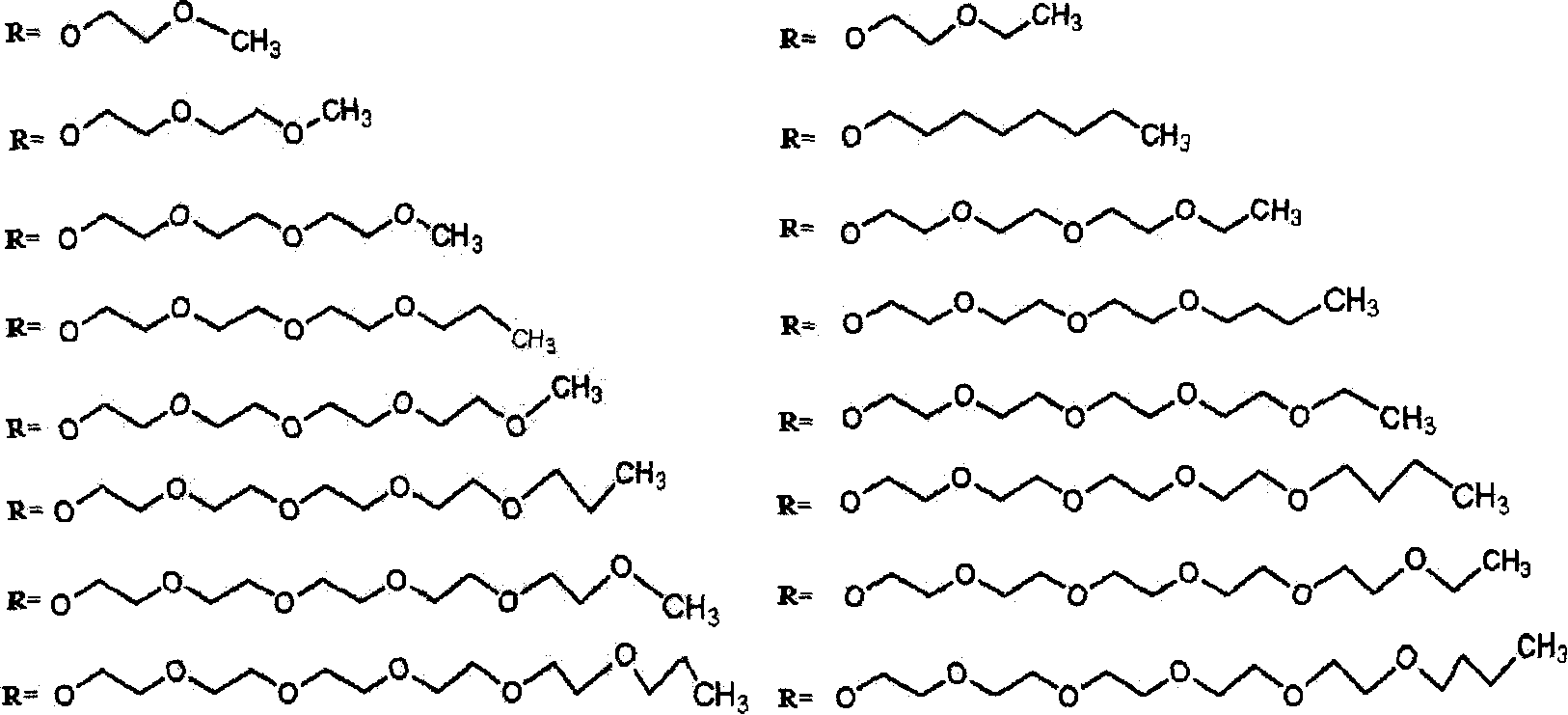
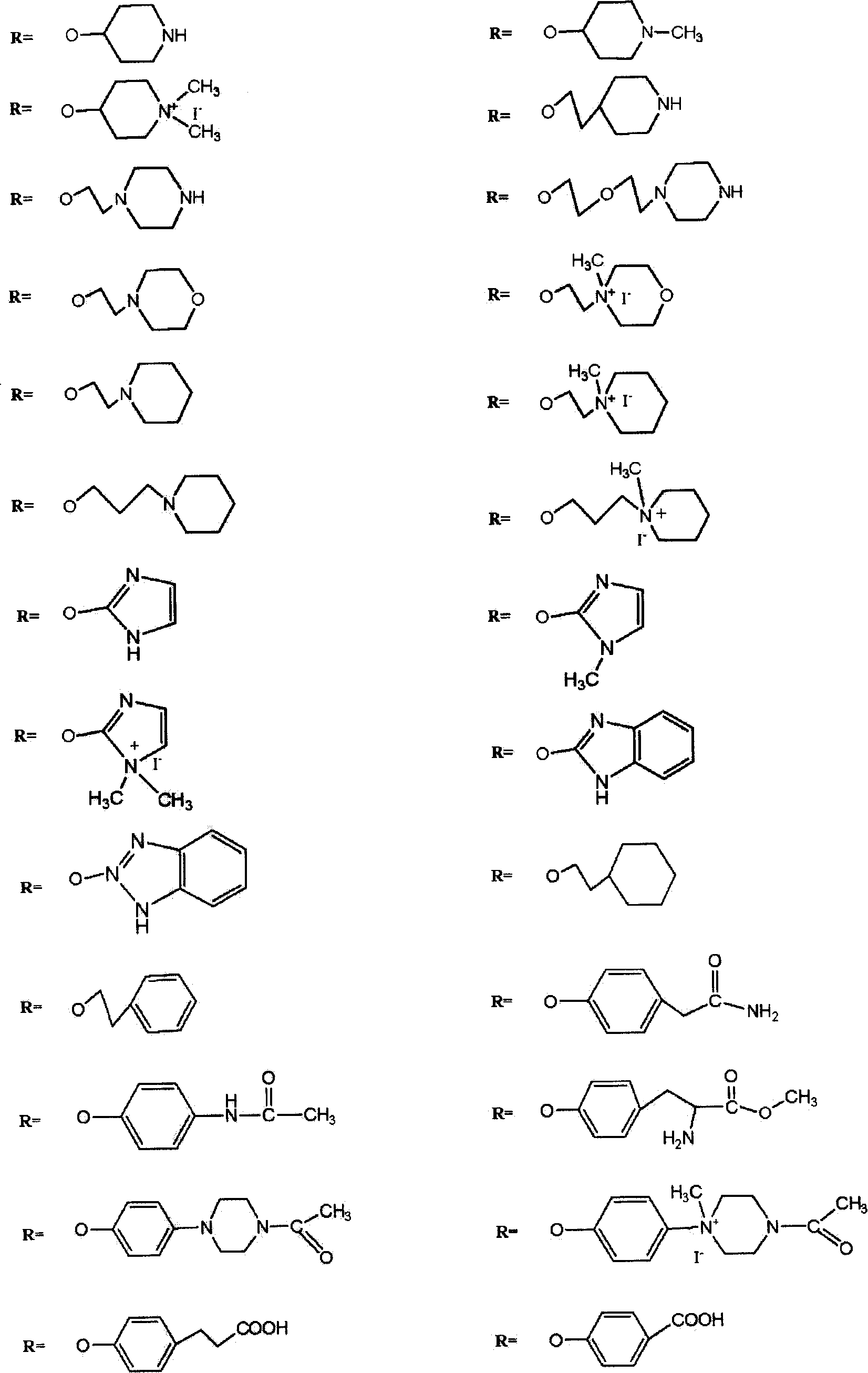
Silicon phthalocyanine compound and composite, their preparation and application thereof
A technology of complexes and complexes, which is applied in the direction of silicon organic compounds, active ingredients of silicon compounds, medical preparations containing active ingredients, etc., to achieve the effects of clear structure, good biosafety, and strong photosensitive activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0089] The preparation method of the silicon phthalocyanine complex of the present invention is characterized in that: (a) using dichlorosilicon phthalocyanine and the alcohol derivative of the substituting group described in claims 1-2 as reactants, the molar ratio of the two 1:2~10, the reaction solvent is preferably toluene or N, N-diformimide or 2-methoxyethyl ether; (b) the reaction needs to be carried out in the presence of sodium hydride or potassium carbonate, and every mole of dichlorosilane Phthalocyanine needs to add 2-6 moles of sodium hydride or potassium carbonate; (c) at a temperature between 70 °C and the reflux temperature of the reaction solvent, react for 3 hours to 36 hours; (d) after the reaction is completed, by solvent method or Column chromatography or high performance liquid chromatography to remove excess raw materials and impurities and purify the target product.
[0090] The preparation method of the silicon phthalocyanine complex of the present inv...
Embodiment 1
[0098] Synthesis and physicochemical properties of two [(2-methoxy) ethoxy] silicon phthalocyanines: Dichloro silicon phthalocyanines and ethylene glycol monomethyl ether are charged according to the molar ratio of 1: 2~10 (preferably 1: 4) than dispersed in anhydrous toluene solvent. In the presence of sodium hydride (2 to 6 moles of sodium hydride, preferably 3 moles, should be added per mole of dichlorosilicon phthalocyanine), react at reflux temperature for 3 hours to 36 hours (preferably 12 hours). After the reaction was complete, the solvent was removed by rotary evaporation under reduced pressure, washed with water, filtered, the filter cake was separated and purified by silica gel chromatography, the main components were collected, and the solvent was evaporated to dryness to obtain the target product as a dark blue solid with a yield of 29%. The maximum absorption peak of the UV-Vis spectrum of the product is 672nm (in DMF solution), mass spectrum (ESI) m / z: 713[M+Na]...
Embodiment 2
[0101]Synthesis and physicochemical properties of two [(2-ethoxy) ethoxy] silicon phthalocyanine: dichloro silicon phthalocyanine and ethylene glycol monoethyl ether according to the feed molar ratio of 1: 2~10 (preferably 1: 4) Dispersed in anhydrous toluene solvent. In the presence of sodium hydride (2 to 6 moles of sodium hydride, preferably 3 moles, should be added per mole of dichlorosilicon phthalocyanine), react at reflux temperature for 3 hours to 36 hours (preferably 12 hours). After the reaction was complete, the solvent was removed by rotary evaporation under reduced pressure, washed with water, filtered, the filter cake was separated and purified by silica gel chromatography, the main components were collected, and the solvent was evaporated to dryness to obtain a dark blue solid as the target product. The maximum absorption peak of the UV-Vis spectrum of the product is 672nm (in DMF solution), mass spectrum (ESI) m / z: 741[M+Na] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com