Prodrug of taxol or polyene-taxol with carbowax as carrier
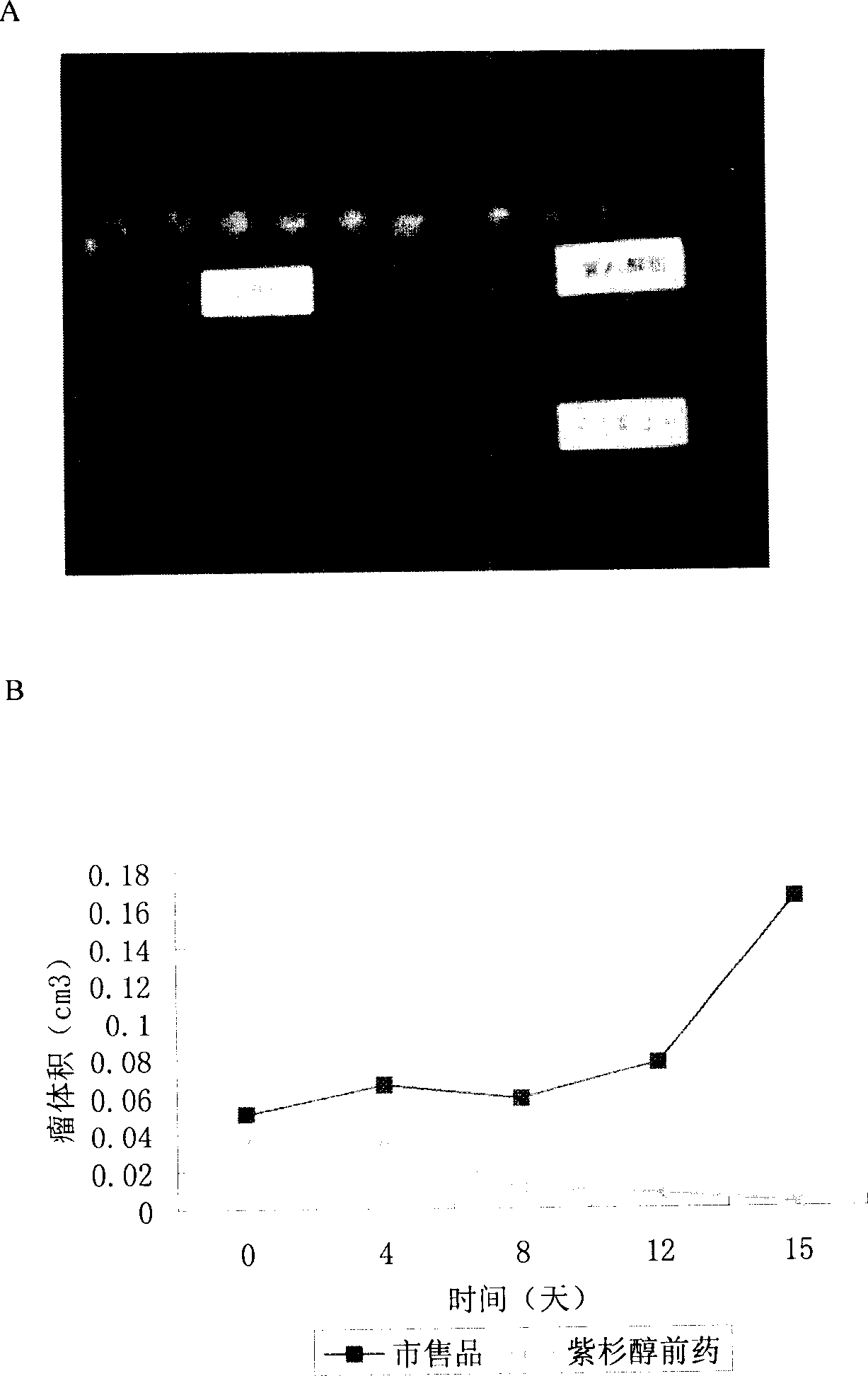
A technology of docetaxel and polyethylene glycol, which is applied in the field of water-soluble paclitaxel or derivative compounds of docetaxel, which can solve the problems of large toxic side effects and inconvenient application, and achieve the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
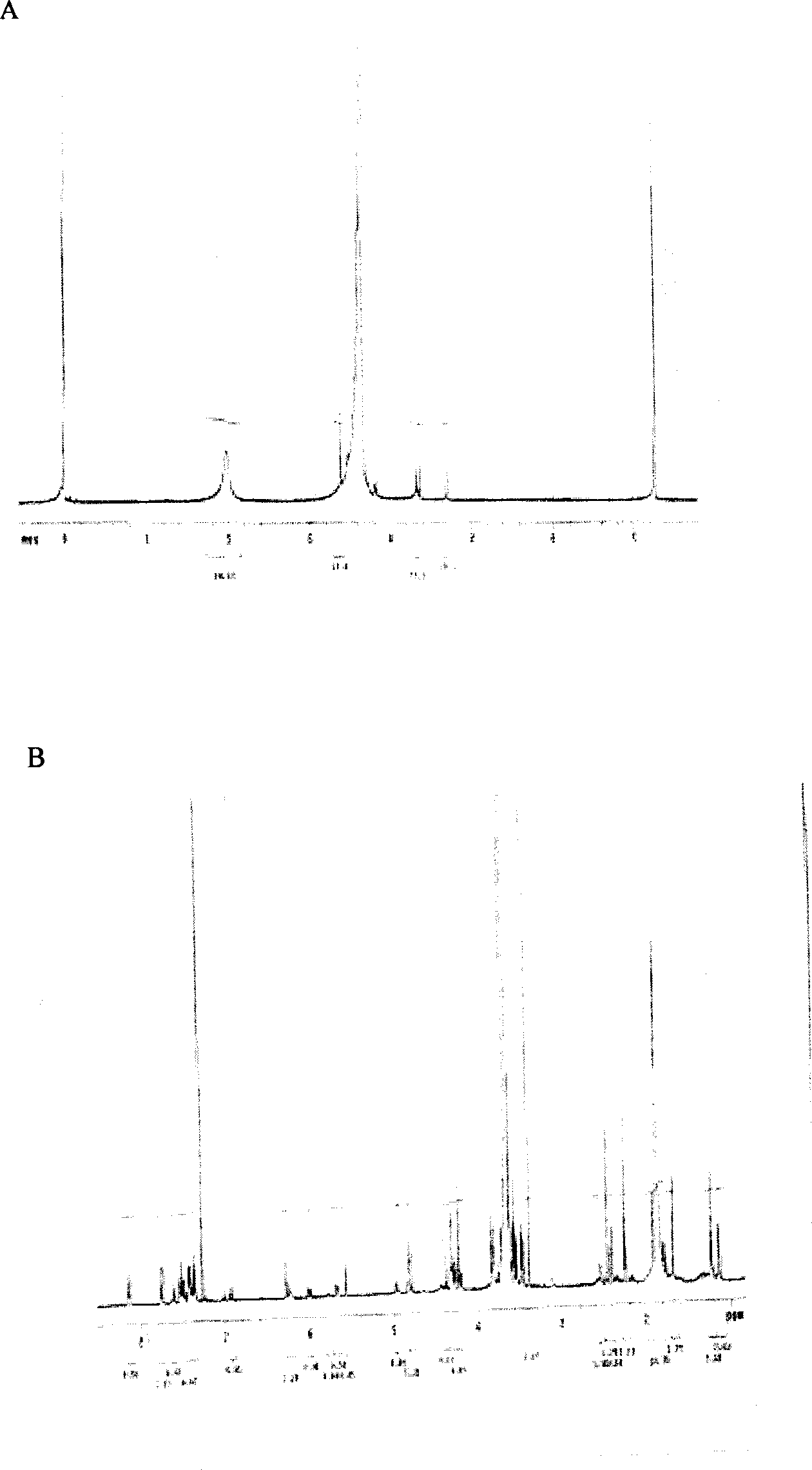
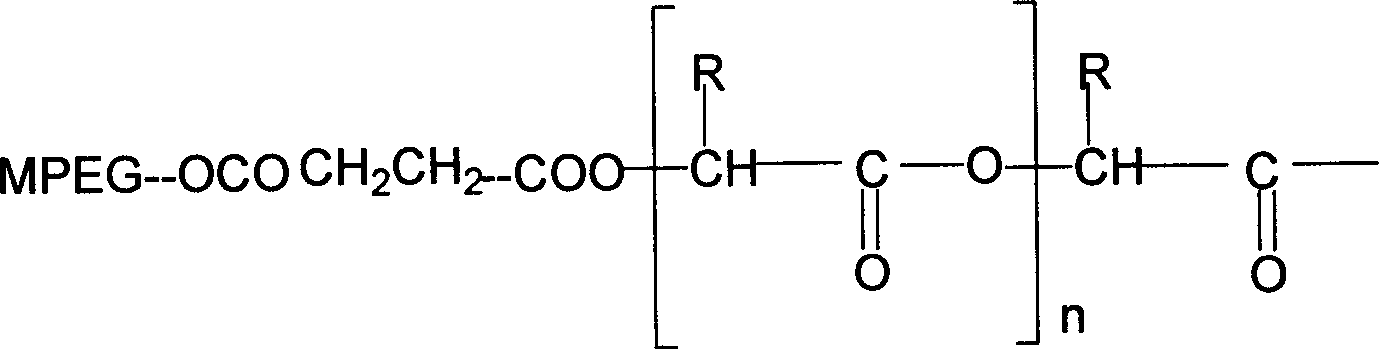
[0039] Example 1: Synthesis of polyethylene glycol 5000-succinic acid-glycolic acid-paclitaxel
[0040] 1. Functionalization of methoxy-terminated polyethylene glycol
[0041] In a 100ml there-necked flask, equipped with a reflux condensing device and a magnetic stirrer, the baking reaction device is ventilated three times (high-purity argon), under gas protection, add 10g of polymer and 1g of pre-weighed succinic anhydride, and An equimolar amount of dimethylaminopyridine (DMAP) was added to 60 ml of anhydrous and oxygen-free tetrahydrofuran, and the mixture was refluxed for 72 hours. After completion of the reaction, concentrate the solvent, then add 10ml of 0.1M aqueous sodium bicarbonate solution to the residue, acidify the filtrate with 10ml of 0.1N hydrochloric acid, adjust the pH value of the solution to 3-4 with hydrochloric acid, and then use 100ml of ethyl acetate to Wash the water layer three times with ester, combine the ethyl acetate solution and wash twice with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com