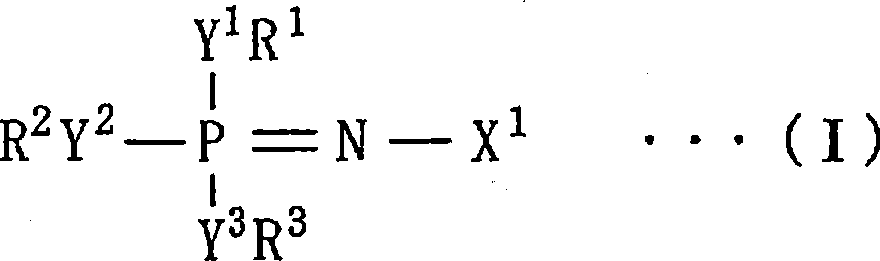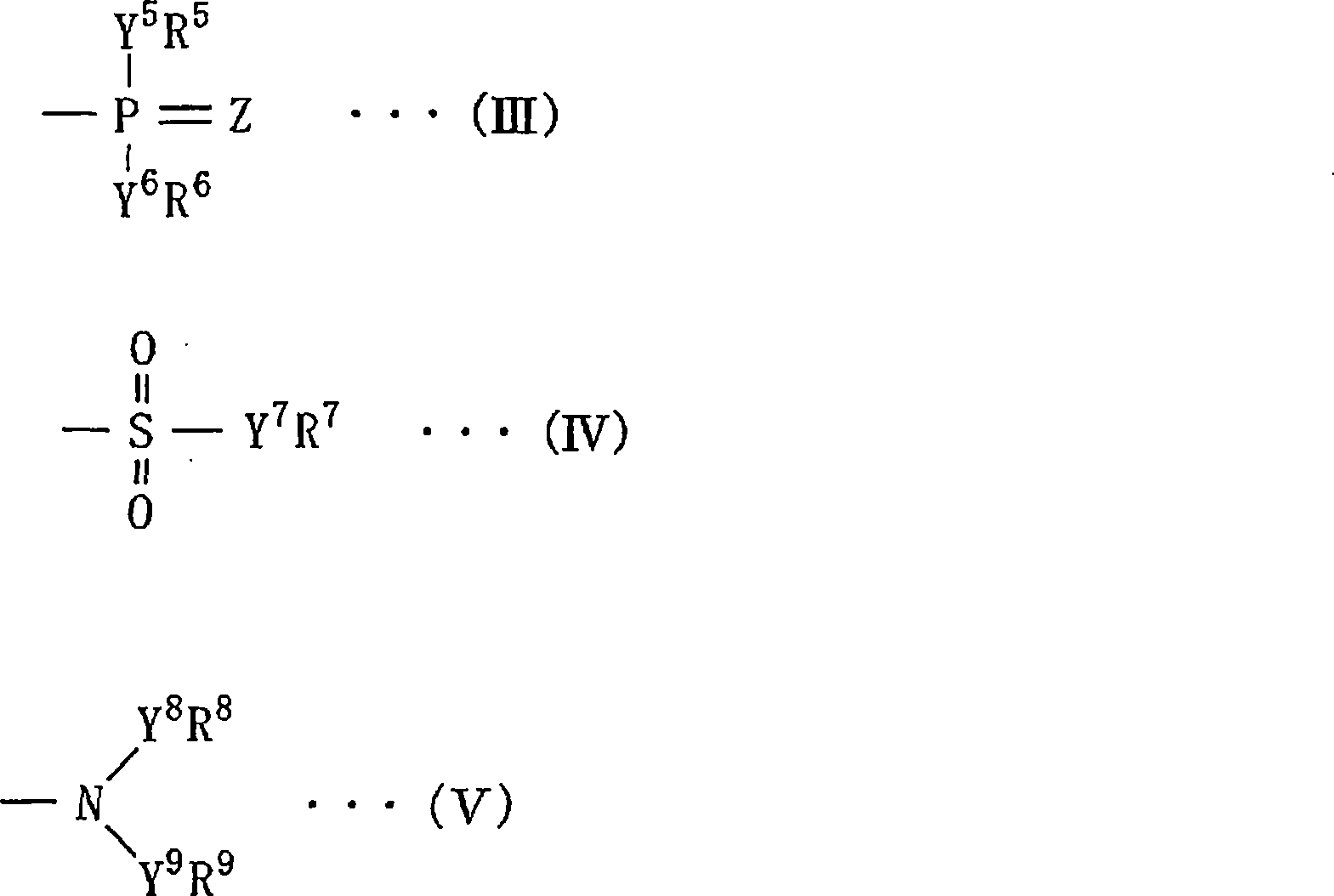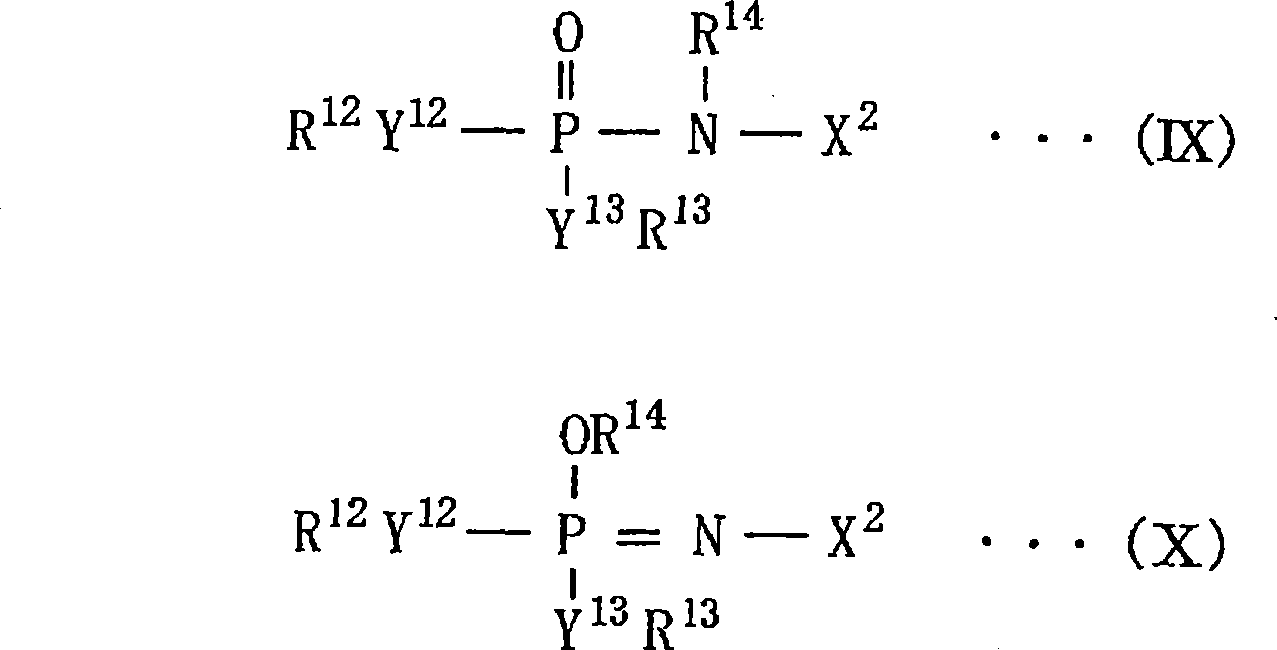Nonaqueous liquid electrolyte for battery, nonaqueous liquid electrolyte battery, electrolyte for polymer battery and polymer battery
A non-aqueous electrolyte and polymer technology, applied in the direction of non-aqueous electrolyte batteries, non-aqueous electrolyte batteries, organic electrolyte batteries, etc., can solve the problems of high risk of battery rupture, fire, spark ignition, etc., to reduce the risk, risk reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] The method for preparing the electrolyte for polymer batteries of the present invention is not particularly limited. For example, the following preparation method can be mentioned: mixing the above-mentioned polymer and supporting electrolyte at a mass ratio (polymer / supporting electrolyte) of 9 / 1 , add volatile solvent, mix well, make it dissolve uniformly at about 80°C, heat to about 40°C in vacuum, volatilize volatile solvent, impregnate and swell aprotic organic solvent and containing phosphorus and / or nitrogen after drying The mixed solution of the compounds to prepare the electrolyte. Examples of the volatile solvent include acetonitrile, alcohols, and the like, and acetonitrile is preferable in terms of excellent solubility and the like.
[0088] As the form of the polymer battery electrolyte of the present invention, it is preferable to impregnate and swell a mixed solution of an aprotic organic solvent and a compound containing phosphorus and / or nitrogen in the...
Embodiment 1
[0103] Prepare by 50 volume % ethylene carbonate (EC, boiling point 238 ℃), 40 volume % diethyl carbonate (DEC, boiling point 127 ℃), 5 volume % additive A [n is 3, 6 in the general formula (II) R 4 3 of them are methoxy (CH 3 O-), 3 cyclic phosphazene compounds that are fluorine, viscosity at 25°C: 3.9mPa·s, boiling point 230°C], 5% by volume additive B [n is 3, 6 R in the general formula (II) 4 One of them is ethoxy (CH 3 CH 2 O-), five cyclic phosphazene compounds that are fluorine, viscosity at 25°C: 1.2mPa·s, boiling point 125°C], and dissolve LiPF in the mixed solution 6 (supporting electrolyte), the concentration is 1mol / L (M), and the non-aqueous electrolyte is prepared. The safety of the obtained non-aqueous electrolytic solution was evaluated by the following method. The results are shown in Table 1.
[0104] (1) Safety of non-aqueous electrolyte
[0105] The safety of the non-aqueous electrolyte was evaluated based on the combustion behavior of the flame that...
Embodiment 2~9 and comparative example 1~6
[0113] Prepare the mixed solution of the proportion shown in table 1 and table 2, dissolve LiPF in this mixed solution 6(supporting electrolyte), the concentration is 1mol / L (M), and the non-aqueous electrolyte is prepared. The safety of the obtained non-aqueous electrolytic solution was evaluated in the same manner as in Example 1. Moreover, similarly to Example 1, the nonaqueous electrolyte solution secondary battery was produced using this nonaqueous electrolyte solution, and the acupuncture test and the overcharge test were implemented for this battery. The results are shown in Table 1 and Table 2.
[0114] In Table 1 and Table 2, PC represents propylene carbonate (boiling point 242°C), DMC represents dimethyl carbonate (boiling point 90°C), and EMC represents ethyl methyl carbonate (boiling point 108°C). , MF represent methyl formate (boiling point: 32°C).
[0115] In addition, additive C is in general formula (II), n is 4, 8 R 4 A cyclic phosphazene compound that is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com