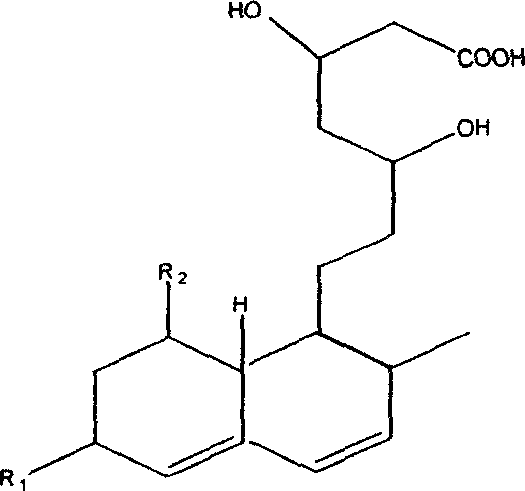
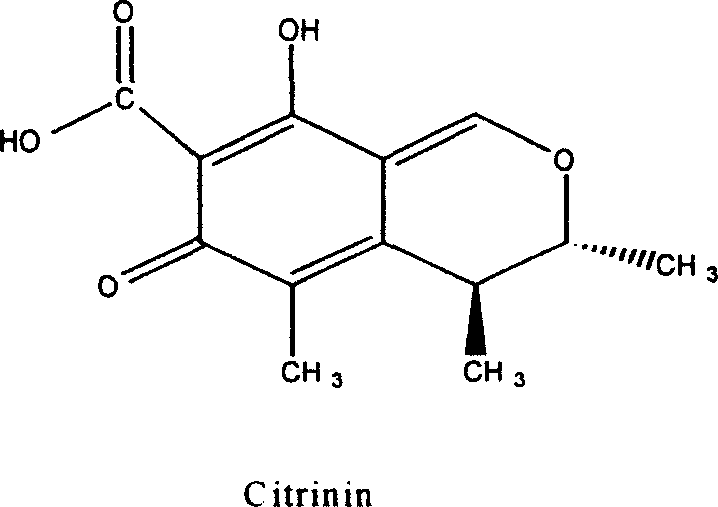
Monascus novel strain and traditional Chinese medicine monascus prepared by fermenting the same
A new strain and technology of red yeast rice, applied in fermentation, fungi, drug combination, etc., can solve the problems of high ring-opening ratio and high polyunsaturated fatty acid content, and achieve high ring-opening ratio, high polyunsaturated fatty acid content, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 The preparation of Chinese medicine lipid-lowering red yeast rice of the present invention: (numbering A)
[0046] The bacterial classification used in the present invention is obtained from soil isolation, identified as purple Monascus (Monascus Purpureus Went) bacterium by Sichuan University Institute of Bioengineering, and preserved by China Typical Culture Collection Center (CCTCC), and the preservation number is CCTCC M 205073.
[0047] After the batch of working seed strains are opened, cultivate them on PDA agar medium plate and store them on the slope of the PDA test tube. , Observing under the microscope after Gram staining, the seeds with the same morphology under the microscope and no bacterial contamination are qualified seeds. Production capacity inspection is to detect the production capacity of the strain (ie the open-ring content per milliliter of fermentation broth) by fermentation at the laboratory level, and any production capacity great...
Embodiment 2
[0050] Embodiment 2 The preparation of Chinese medicine lipid-lowering red yeast rice of the present invention:
[0051] The bacterial classification used in the present invention is obtained from soil isolation, identified as purple Monascus (Monascus Purpureus Went) bacterium by Sichuan University Institute of Bioengineering, and preserved by China Typical Culture Collection Center (CCTCC), and the preservation number is CCTCC M 205073.
[0052] After the batch of working seed strains are opened, cultivate them on PDA agar medium plate and store them on the slope of the PDA test tube. , Observing under the microscope after Gram staining, the seeds with the same morphology under the microscope and no bacterial contamination are qualified seeds. Production capacity inspection is to detect the production capacity of the strain (ie the open-ring content per milliliter of fermentation broth) by fermentation at the laboratory level, and any production capacity greater than 1ug / ml...
Embodiment 3
[0055] Embodiment 3 The preparation of Chinese medicine lipid-lowering red yeast rice of the present invention:
[0056] After the batch of working seed strains are opened, cultivate them on PDA agar medium plate and store them on the slope of the PDA test tube. , Observing under the microscope after Gram staining, the seeds with the same morphology under the microscope and no bacterial contamination are qualified seeds. Production capacity inspection is to detect the production capacity of the strain (ie the open-ring content per milliliter of fermentation broth) by fermentation at the laboratory level, and any production capacity greater than 1ug / ml is a qualified seed]. As seeds for production, the seeds are cultivated at 30-40° C., 180-220 rpm, in PDA medium for 8-24 hours as first-class seeds, and can also be used as fermenter seed liquid.
[0057] Components of solid-state fermentation nutrient solution (g / L): glycerin 150, peptone 150, beef extract 80, Na 2 HPO 4 12H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Decomposition point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com