Application of cumarin kind compound in preparation of medicine for inducing nerve stem cell orienting differentiation
A neural stem cell and coumarin technology, which is applied in the application field of coumarin compounds in the preparation of drugs for inducing neural stem cell directional differentiation, can solve the problem of staying, unable to be used in clinic, and unable to obtain a large number of oligodendrocytes. somatic cell problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
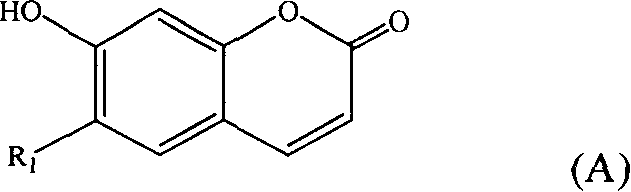
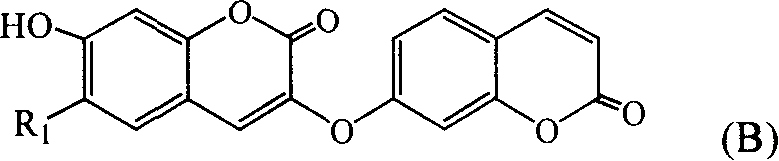
[0028] Embodiment 1: Preparation of 7-hydroxycoumarin and double daphnetin
[0029] 11Kg of Daphne giraldii Nitsche. The root bark and stem bark of Daphne giraldii Nitsche. were crushed and extracted three times with 95% ethanol under reflux, the extracts were combined, the ethanol was recovered under reduced pressure to a volume of 10L, filtered, and the filtrate was washed with petroleum ether and chloroform in turn , ethyl acetate and n-butanol extraction, each extraction part is collected and concentrated into an extract, wherein 200 g of the extract part extracted by ethyl acetate is dissolved in methanol and filtered and then subjected to silica gel column chromatography, and gradient elution is carried out with chloroform-methanol. The eluted fractions were checked by thin-layer chromatography, and the fractions with the same single spot were combined and concentrated to obtain fractions 1, 2, 3, 4, 5, 6, 7, 8, and 9, of which fraction 2 was further subjected to a silica...
Embodiment 2
[0034] Embodiment 2: preparation scopoletin
[0035] 5Kg root bark of Daphne odora Thunb.var.atrocaulis Rehd., crushed and extracted by percolation with 75% ethanol to obtain 50L of extract, which was concentrated and then extracted with petroleum ether, chloroform, ethyl acetate and n-butanol , the extract is concentrated into an extract. The chemical components of each extraction part were systematically separated and identified by silica gel column chromatography, polyamide chromatography, Sephadex LH-20 and preparative HPLC. Twelve compounds were obtained by separation of chloroform, and one of them was scopoletin as light yellow powder after structural identification.
[0036] The structural formula of scopoletin is as follows:
[0037]
Embodiment 3
[0038] Embodiment 3: preparation of acarina
[0039] 5Kg of the rhizome of the medicinal material Azalea japonica is crushed and soaked in 80% ethanol for 24 hours, the soaking liquid is collected, concentrated until it has no alcohol smell, diluted with an appropriate amount of water to form a liquid extract, and extracted with petroleum ether, chloroform, ethyl acetate and n-butanol in sequence, Collect each extraction site and concentrate into an extract. Systematic chemical composition analysis was carried out on the extracts of various parts. As a result, 12 compounds were separated from the chloroform part. After structural identification, one of the light yellow powders was acarina.
[0040] The structure of agarose is as follows:
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com