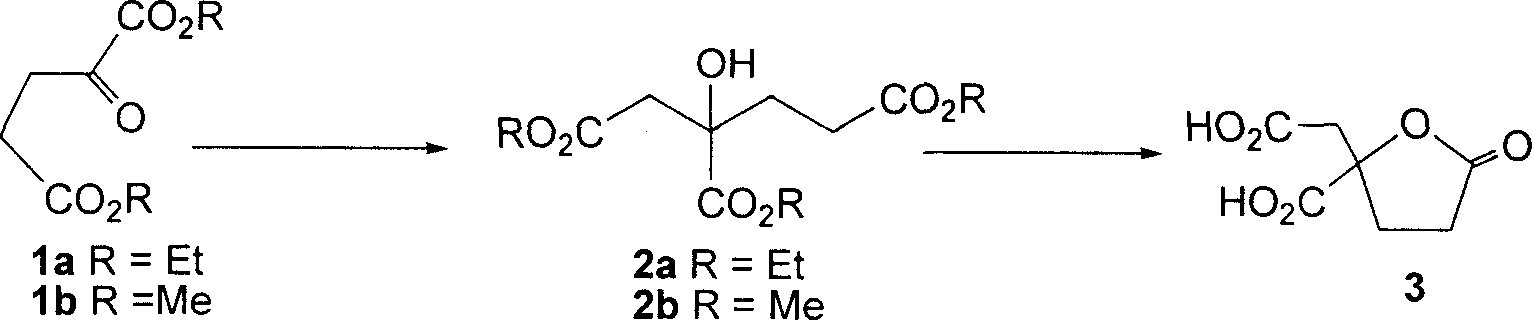
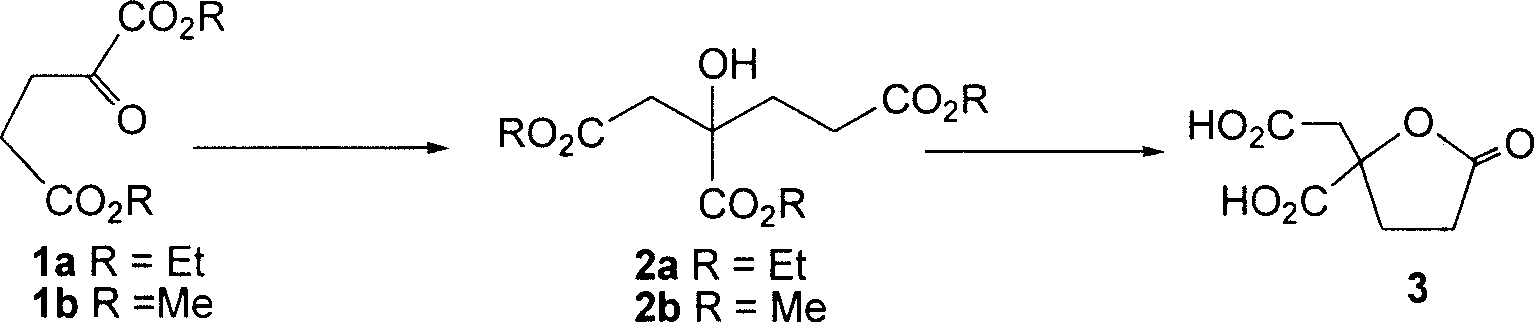
Preparation method of racemic homocitric acid lactone
A citric acid, racemic technology, applied in the direction of organic chemistry and the like, can solve problems such as unsuitable scale preparation, and achieve the effects of high reaction yield, good application prospect and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of triethyl homocitrate 2a: Add n-BuLi (2.5M solution in n-hexane, 1.42mmol, 0.57ml) to a solution of HMDS (0.35ml, 1.65mmol) in THF (1ml) under ice-cooling, After reacting for 30min, cool to -78°C, add ethyl acetate (0.37ml, 1.42mmol), react for 30min, add a solution of 1a (238mg, 1.18mmol) in THF (3.5ml), react for 3h, add saturated NH 4 Quenched with Cl, extracted with ether, combined the organic phases, washed with saturated brine, and dried over anhydrous sodium sulfate. After filtration and concentration under reduced pressure, flash column chromatography (ethyl acetate:petroleum ether=1:6~1:4) gave compound 2a with a yield of 73%. IR(film)ν max : 3505, 2982, 2938, 1736, 1446, 1373, 1191cm -1 ; 1 HNMR (400MHz, CDCl 3 )δ: 2.68(d, J=16.2Hz, 1H), 2.94(d, J=16.2Hz, 1H), 1.31(t, J=7.1Hz, 3H), 1.25(t, J=7.1Hz, 3H) , 1.25(t, J=7.1Hz, 3H), 3.77(s, 1H-OH), 4.13(qq, overlapped, J=7.1Hz, 4H), 4.22~4.31(m, 2H), 2.02~2.07(m , 2H), 2.21~2.29(m, 1H), 2.46~2.54(m,...
Embodiment 2
[0025] Compound 2a was prepared from compound 1a according to the method of Example 1.
[0026] Compound 3 was prepared from compound 2a according to the method of Example 1, using trifluoroacetic acid instead of formic acid, and the rest of the operations were the same, with a yield of 91%.
Embodiment 3
[0028] Compound 2a was prepared from compound 1a according to the method of Example 1, and the molar ratio of base and ester was 1:2.
[0029] Compound 3 was prepared from compound 2a according to the method of Example 1, trifluoroacetic acid was added to formic acid, the ratio of formic acid to trifluoroacetic acid was 1:1, the molar ratio of compound 2a and acid was 1:10, and the rest of the operations were the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com