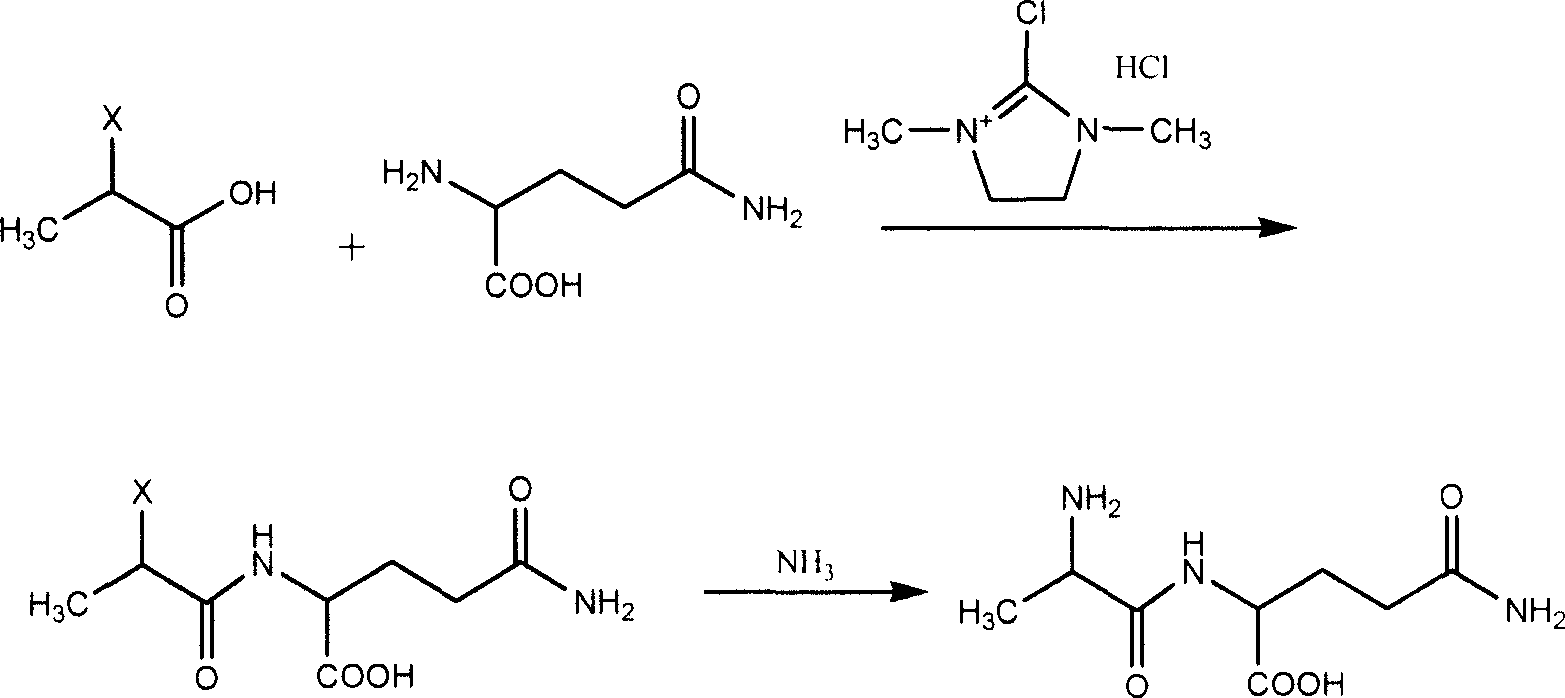
Prepn process of L-alanyl-L-glutamine
A technology of halogenated propionyl glutamine and glutamine, which is applied in the field of preparation of L-alanyl-L-glutamine, can solve the production cost of human hazards, L-alanyl-L-glutamine Problems such as the complex preparation process of amide, to achieve the effect of being beneficial to environmental protection, good industrial application value, and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]D-2-chloropropionic acid (35.8 g, 0.33 mol) was dissolved in 200 mL of benzene at room temperature, and the condensing agent 2-chloro-1,3-dimethylimidazoline hydrochloride (61.2 g, 0.36 mol), added glutamine (46.9 g, 0.33 mol) after 4 hours of reaction, filtered after 2 hours of reaction, washed with 5 wt% hydrochloric acid and water to obtain D-2-halopropionylglutamine. The obtained product was directly added to 28 wt% ammonia water (800 ml), reacted at 75° C. for 6 hours under a pressure of 2 kg / cm 2 , cooled, concentrated under reduced pressure, added with ethanol, and left to separate out crystals to obtain 47.6 g of L- Alanyl-L-glutamine product, yield 66.5%; optical purity 98.5%.
[0022] The above product was recrystallized from methanol-water (volume ratio 2:1) to obtain L-alanyl-L-glutamine product with an optical purity of 99.8%, melting point: 216°C (decomposition); specific optical rotation: [α] 20 =-3.49 (C=10, 1 mol / L HCl).
Embodiment 2
[0024] At room temperature, D-2-bromopropionic acid (61.2 g, 0.4 mol) was dissolved in 300 mL of toluene, 2-chloro-1,3-dimethylimidazoline hydrochloride (68.0 g, 0.4 mol) was added ), after 4 hours of reaction, glutamine (49.7 g, 0.35 mol) was added, filtered after 2 hours of reaction, washed with 5wt% hydrochloric acid and water to obtain D-2-bromopropionylglutamine. The obtained product was directly added to 28% by weight ammonia water (1000 ml), reacted at 45° C. for 6 hours at 2 kg / cm 2 , cooled, concentrated under reduced pressure, added with ethanol, and left to separate out crystals to obtain 55.9 g of L-propane. Aminoacyl-L-glutamine product, yield 73.6%, optical purity 98.8%.
[0025] The above product was recrystallized from ethanol-water (volume ratio 5:1) to obtain L-alanyl-L-glutamine product with an optical purity of 99.9%, melting point: 216°C (decomposition); specific optical rotation: [α] 20 =-3.45 (C=10, 1 mol / L HCl).
Embodiment 3
[0027] D-2-chloropropionic acid (32.6 g, 0.30 mol) was dissolved in 250 mL of toluene at room temperature, 2-chloro-1,3-dimethylimidazoline hydrochloride (42.5 g, 0.25 mol) was added ), added glutamine (42.6 g, 0.30 mol) after 5 hours of reaction, filtered after 2 hours of reaction, washed with 5 wt% nitric acid and water to obtain D-2-halopropionyl glutamine. The obtained product was directly added to 28% by weight ammonia water (900 ml), reacted at 60° C. for 6 hours under a pressure of 3 kg / cm 2 , cooled, concentrated under reduced pressure, added with methanol, and left to separate out crystals to obtain 42.9 g of L- Alanyl-L-glutamine product, yield 65.9%; optical purity 97.8%.
[0028] The above product was recrystallized from tetrahydrofuran-water (volume ratio 1:2) to obtain L-alanyl-L-glutamine product with an optical purity of 99.8%, melting point: 216°C (decomposition); specific optical rotation: [α] 20 =-3.40 (C=10, 1 mol / L HCl).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com