Compositions and methods for modifying the content of polyunsaturated fatty acids in biological cells
A fatty acid and substance technology, applied in the field of compositions for changing the content of polyunsaturated fatty acids in animal cells, can solve problems such as lack of 12- and 15-dehydrogenase activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: Construction of recombinant adenovirus
[0081] The recombinant adenovirus carrying the fat-1 gene was constructed following the steps similar to those described by He et al. (please refer to Proc. Natl. Acad. Sci. USA95: 2509-2514, 1998). The n-3 fatty acid dehydrogenase cDNA (fat-1 gene) in pCE8 was kindly provided by Dr. J. Browse (Washington State University) (but can also be synthesized or cloned using common techniques and information in the art; please refer to Spychalla et al. al., Proc. Natl. Acad. Sci. USA 94:1142-1147, 1997; US Pat. No. 6,194,167; and refer to FIGS. 17A and 17B). The cDNA insert gene of pCE8 was excised from the plasmid by restriction enzyme EcoRI / Kpnl double shearing reaction and inserted into the transfer vector (shutter vector), and then the adenovirus backbone was recombined according to the method of He et al. (supra). Two first-generation recombinant adenoviruses of type 5 were formed: Ad.GFP carrying green fluorescent prote...
Embodiment 2
[0083] Example 2: Adenovirus culture and infection of cardiomyocytes
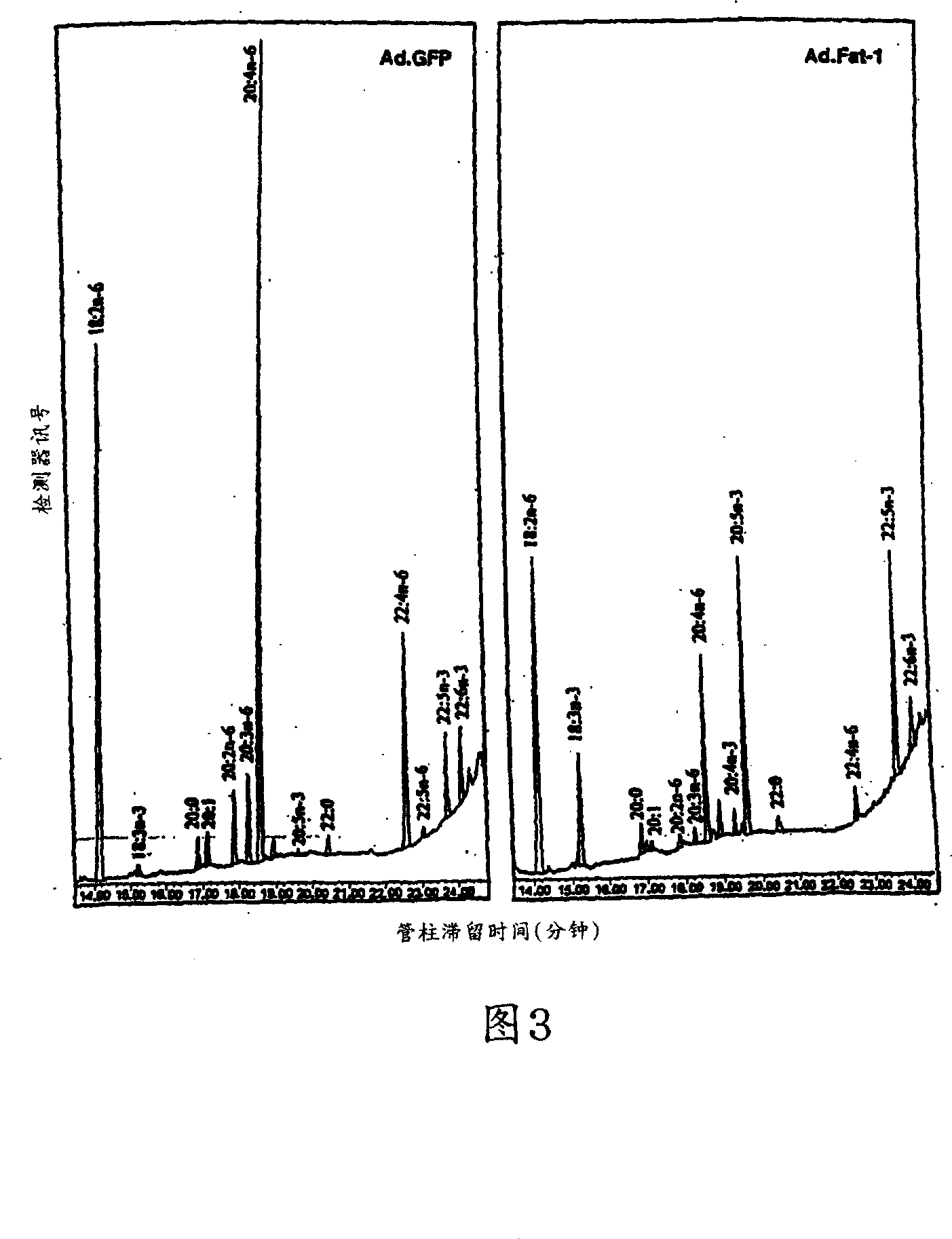
[0084] Cardiomyocytes were isolated from one day old mice using the National Cardiomyocyte Isolation System (provided by Worthington Biochemical Corp., Freehold, NJ). Then place it in a 6-well plate, place it in a tissue culture machine (containing 5% CO) in F-10 culture medium (containing 5% fetal bovine serum, 30% horse serum, 2 and 98% relative humidity) for cultivation. Cells used in experiments need to be cultured for 2-3 days. Add different concentrations (5*10 9 -10 10 pfu) of virus particles. After culturing for 24 hours, 10 μM of 18:2n-6 and 20:4n-6 were added to the culture medium. Forty-eight hours after infection, the cells can be used (eg, for analysis of gene expression, fatty acid composition, viability, or growth capacity (eg, proliferation or division rate)).
Embodiment 3
[0085] Example 3: Detection of fat-1 expression and RNA analysis by fluorescence microscopy
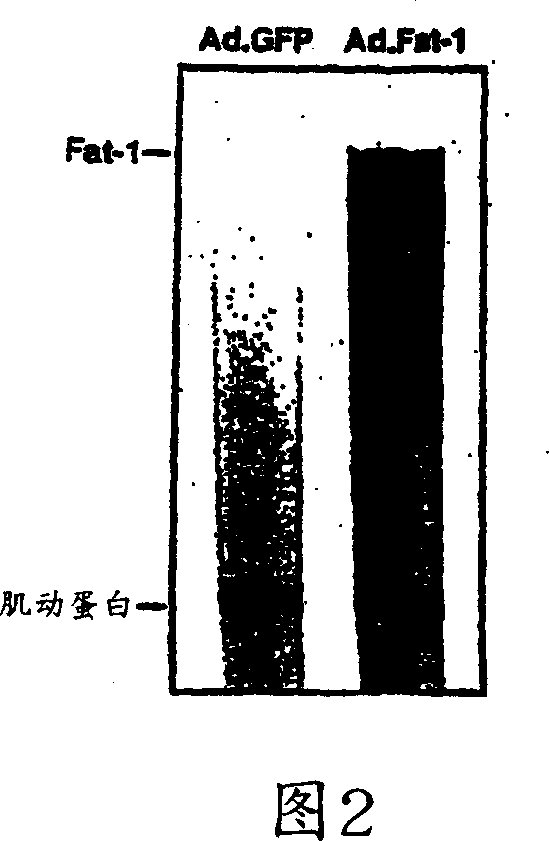
[0086] There are many well-known methods in the field of molecular biology to detect gene expression. Here, the expression of fat-1 in cardiomyocytes after the above-mentioned infection steps was measured by visual detection of infected cells and RNase enzyme protection assay.
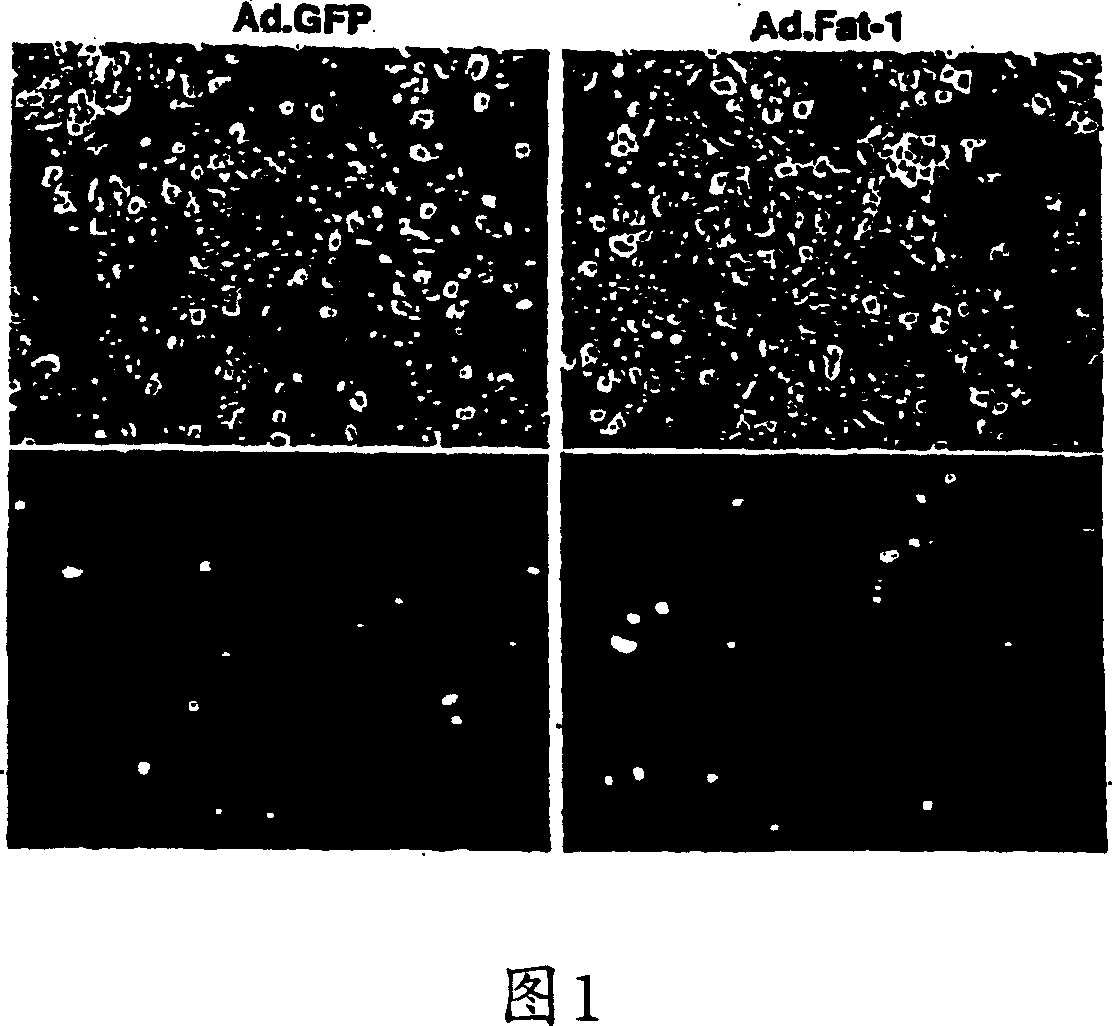
[0087] In detail, GFP co-expression was used to identify infected cells as well as cells expressing the transgene. Approximately 48 hours after infection, almost all cells (>90%) showed bright fluorescence, indicating a high gene transfer rate and a high expression level of the transgene (see Figure 1). Expression of Fat-1 gene transcripts can also be performed using RPA III TM The equipment (provided by Ambion) was determined by the RNase enzyme protection test method. Briefly, total RNA was extracted from cultured cells using RNA extraction equipment (provided by Qiagen) according to the manufacturer's pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com