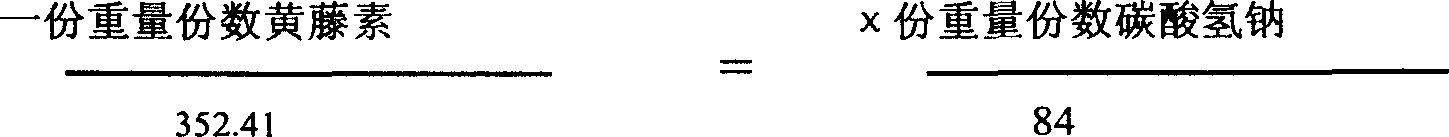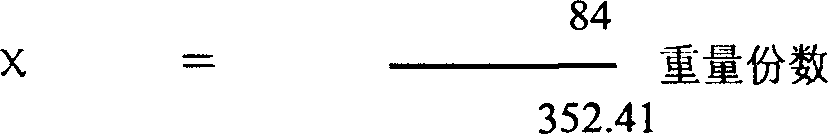Derivative of fibrauretine, and preparation method
A technology of palmetine and its derivatives, which is applied in the field of palmetine derivatives and its preparation, can solve the problems of affecting clinical efficacy and clinical application range, low bioavailability of oral preparations, limiting administration routes and dosage forms, etc., achieving Improved bioavailability, improved water solubility and absorbability, and low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Select 1 kg of pure palmatine, and another 0.238 kg of pure sodium bicarbonate (that is, the same molar quantity), add water for injection and dilute to 212 liters, mix and place in a water bath at 55°C until completely dissolved to obtain an orange-yellow to wine 212 liters of red clear solution can obtain 0.5% solution. Add acid-base buffer to the solution, filter and sterilize it, and pack it into 106,000 2ml injections, each containing about 10 mg of dry product of the new drug.
example 2
[0030] Take 1 kg of pure palmetine, add 80 kg of water for injection, heat it in a water bath at 55°C until it dissolves to obtain a yellow clear liquid, add 0.238 kg of sodium bicarbonate to 20 kg of water for injection until completely dissolved, and mix the two solutions , stir evenly, after the reaction, an orange-red clear liquid is obtained, add an appropriate amount of acid-base buffer pair and a cosolvent (such as Tween-80) to settle to 106 liters to obtain a 1% solution, filter and sterilize, and pack into 53000 5 ml vials placed in a freeze dryer. After vacuum drying at low temperature for 24 to 36 hours, freeze-dried powder injections are obtained, each containing about 20 mg of the new drug.
example 3
[0032] Take 1 kg of pure palmetine, add 80 kg of water for injection, heat it in a water bath at 55°C until it dissolves to obtain a yellow clear liquid, add 0.238 kg of sodium bicarbonate to 20 kg of water for injection until completely dissolved, and mix the two solutions , stirred evenly, after the reaction, an orange-red clear liquid was obtained, adding an appropriate amount of acid-base buffer pair and a cosolvent (such as Tween-80) to constant volume to 106 liters to obtain a 1% solution, and drying or low-temperature drying to obtain a dry product of 1.2 to 1.3 Add dextrin, medicinal starch, etc. after one kilogram of crushing, add distilled water and stir evenly, press into 60,000 to 65,000 tablets and dry, each containing about 20 mg of the drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com