Porcine alpha-interferon recombinant adenovirus and its construction method and application
A technology of recombinant adenovirus and alpha interferon, which is applied in the fields of application, antiviral agent, virus/phage, etc., to achieve the effect of preventing various diseases, inhibiting virus replication, and stabilizing the titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
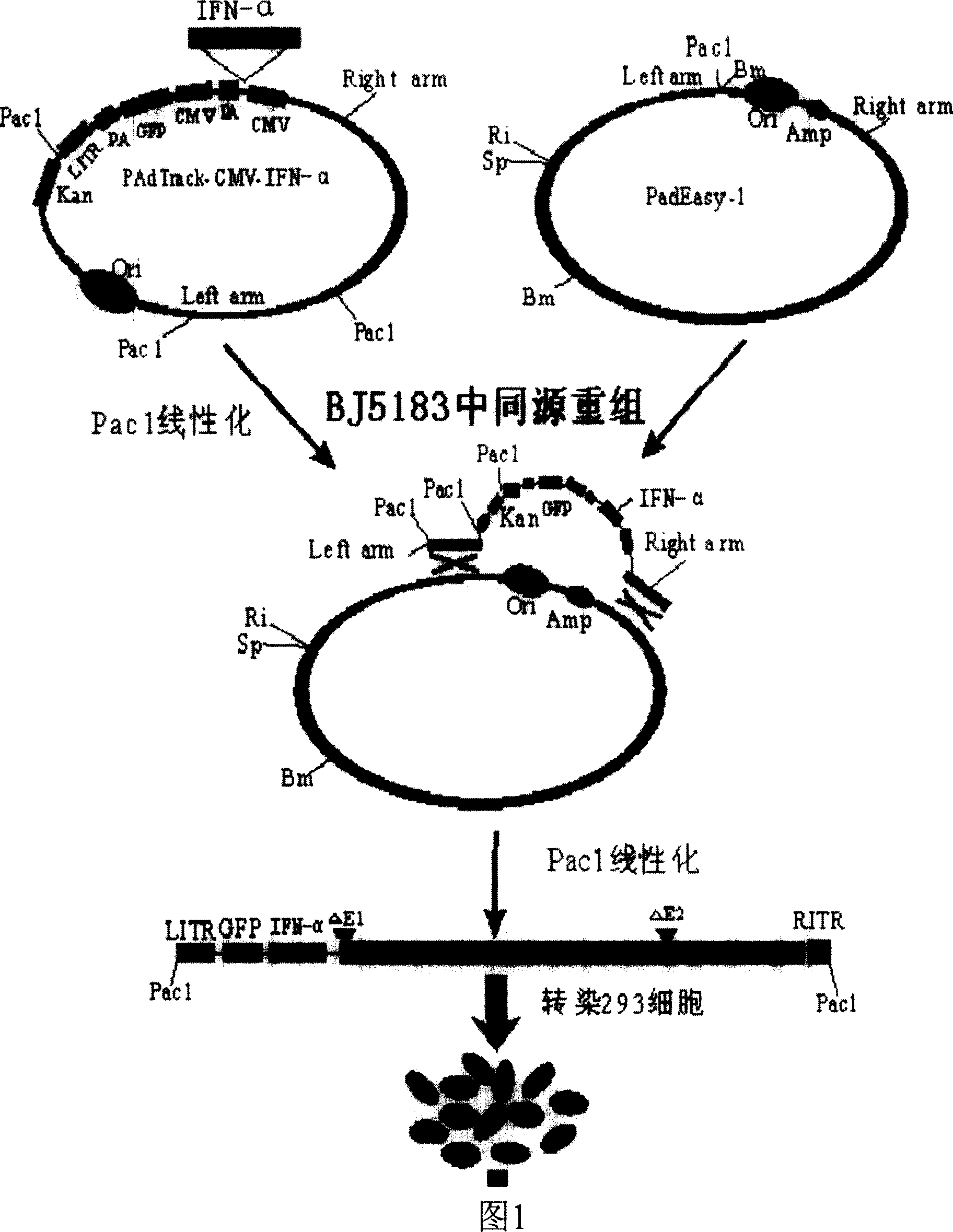
[0033] As shown in FIG. 1 , it is a flow chart of the production process of the recombinant porcine interferon-α adenovirus.
[0034] 1. Construction of porcine alpha interferon recombinant adenoviral plasmid (pAdCMV-IFN-α)
[0035] (1) Primer
[0036] Two pairs of primers were designed according to GenBank porcine interferon alpha IFN-α1 gene sequence (accession number X57191) and related pseudogene sequence (accession number AF350425), wherein primers P1 and primer P2 amplified the first half of the gene (F fragment), primers P3, Primer P4 amplifies the second half (fragment B). There is a 37bp repeat sequence between the two genes of the F segment and the B segment, which can meet the needs of splice overlap extension PCR (splice overlap extension PCR, SOE-PCR). Primer P2 and primer P3 are located in the region where the variation is concentrated within the gene, and their 3' terminal bases are only specifically complementary to the porcine IFN-α gene, but have difference...
Embodiment 2
[0079] Preparation of porcine alpha interferon (IFN-α) recombinant adenovirus vaccine
[0080] The purified porcine interferon-alpha recombinant adenovirus was continuously passaged on 293 cells for 30 times, and the expression of porcine interferon-alpha was detected, which proved that the expression of porcine interferon-alpha was stable, and the toxicity of the recombinant adenovirus was stable at 10 4.56 TCID 50 / 1.0mL.
[0081] During the expanded culture process, the purified and preserved porcine interferon-alpha recombinant adenovirus was used for 10 4 TCID 50 Seed to grow into a monolayer of 293 cells. When the cytopathy reaches 70-90%, the virus is harvested, and the porcine alpha interferon recombinant adenovirus vaccine can be obtained after repeated freezing and thawing once.
Embodiment 3
[0083] Immunizing pigs with porcine interferon-alpha recombinant adenovirus as a vaccine
[0084] The recombinant adenovirus containing porcine alpha interferon (IFN-α) gene has a virulence of 10 after artificial cultivation. 4.56 TCID 50 / 1.0mL, freeze-dried in a vacuum, used as a vaccine, has a good preventive effect on various diseases of pigs. The method of use is that sows and boars are immunized 3 times a year, 4-6 ml each time; small pigs are injected with 1-3 ml intramuscularly on 7-10 days, and 1-3 ml is injected intramuscularly after 10 days; medium pigs and large pigs can be Boost the immunization again, and inject 3 to 6 ml intramuscularly each time. It can effectively prevent various diseases of pigs caused by PCV2, prevent poor semen quality of boars, miscarriage, stillbirth, mummification and small litter size of sows, the effective rate can reach 90%, and prevent multi-system failure of pigs after weaning Syndrome (PMWS) the effective rate can reach more tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com