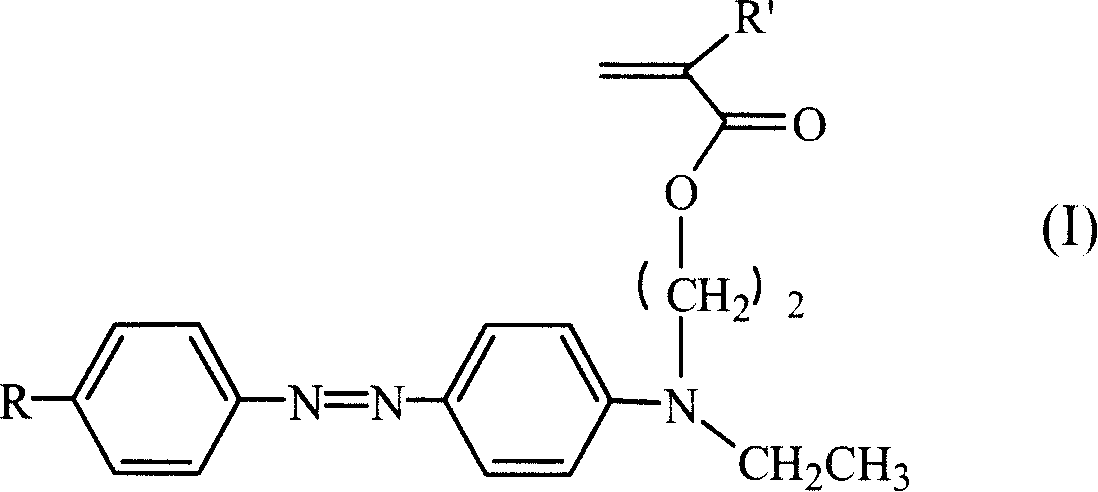
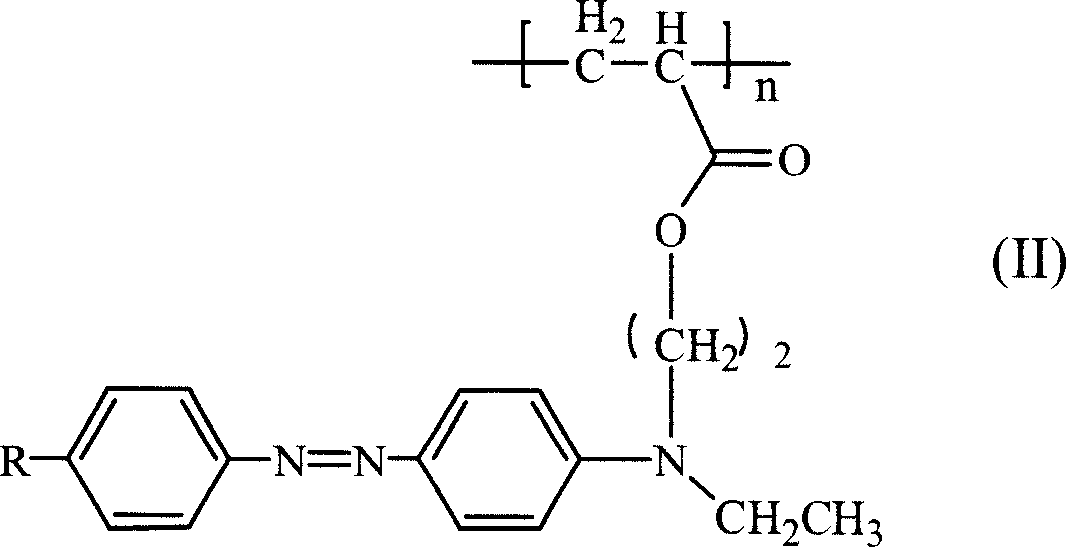
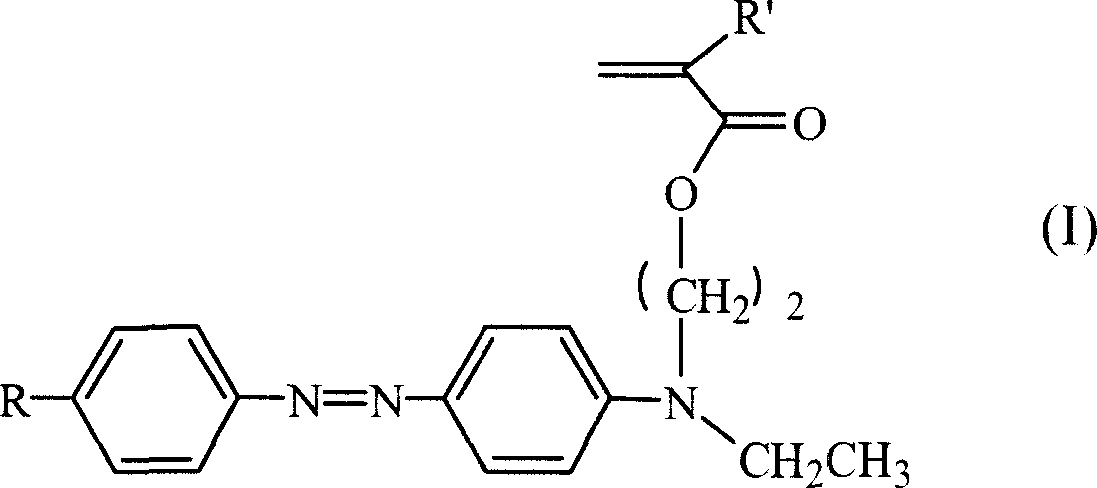
Third order non-linear optical material monomer, its polymer and production
A third-order nonlinear, optical material technology, applied in the field of atom transfer radical polymerization to prepare the polymer, to achieve the effects of good solubility and film-forming properties, controllable molecular weight, and high third-order nonlinear polarizability coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Take 2-3g of p-nitroaniline to make hydrochloride solution, put it in an ice bath to cool, add dropwise 10g of 15-20% sodium nitrite aqueous solution, and react in an ice bath for half an hour to obtain p-nitroaniline diazonium salt solution. Take 3~5g of N,N-ethylhydroxyethylaniline, 3~5g of sodium hydroxide, add 50~100g of water, stir to dissolve, put it in an ice bath to cool, and add diazo at a rate of about one drop every 2-4 seconds Salt solution, continue to react for 0.5 to 1 hour after the dropwise addition, and keep warm for 4 to 8 hours. The reaction solution was suction filtered and washed with a large amount of deionized water to obtain the azo intermediate. The third-order nonlinear susceptibility coefficient of the intermediate is measured by four-wave mixing method to be 6.1~6.3×10 -11 esu.
[0030] 2. Take 3-4 g of the above p-nitroazo intermediate, dissolve it in 40-50 ml of tetrahydrofuran, add 2-3 ml of triethylamine, and stir evenly under ice ...
Embodiment 2
[0034] 1. Take 2-3g of p-methoxyaniline to make hydrochloride, put it in an ice bath to cool, add dropwise 10g of 15-20% sodium nitrite aqueous solution, and react under ice bath for half an hour to obtain p-methoxyaniline diazonium salt solution. Take 3~5g of N,N-ethylhydroxyethylaniline, 3~5g of sodium hydroxide, add 50~100g of water and stir to dissolve, cool in an ice bath, add the above diazonium salt solution dropwise, and continue the reaction for 0.5 ~1 hour, keep warm for 4~8 hours. The reaction solution was suction-filtered and washed with a large amount of deionized water to obtain a p-methoxyazo intermediate. The third-order nonlinear susceptibility coefficient of the intermediate was measured by four-wave mixing method to be 4.2~4.4×10 -11 esu.
[0035] 2. Take 3-4g of p-methoxyazo intermediate, dissolve it in 20-30ml of tetrahydrofuran, add 2-3ml of triethylamine, and stir evenly under ice bath. Take 1-2 g of acryloyl chloride and slowly add it dropwise to th...
Embodiment 3
[0039] 1. Take 2-3g of p-chloroaniline to make a hydrochloride solution, put it in an ice bath to cool, add dropwise 10g of 15-20% sodium nitrite aqueous solution, and react in an ice bath for half an hour to obtain a p-chloroaniline diazonium salt solution. Take 3~5g of N,N-ethylhydroxyethylaniline, 3~5g of sodium hydroxide, add 50~100g of water and stir to dissolve, cool in an ice bath, add the above diazonium salt solution dropwise, and continue the reaction for 0.5 ~1 hour, keep warm for 4~8 hours. The reaction solution was suction filtered and washed with a large amount of deionized water to obtain the p-chloroazo intermediate. The third-order nonlinear susceptibility coefficient of a sample of this intermediate measured by four-wave mixing method is 4.5~4.7×10 -11 esu.
[0040] 2. Take 3-4g of p-chloroazo intermediate, dissolve it in 30-40ml of tetrahydrofuran, add 2-3ml of triethylamine, and stir evenly under ice bath. Take 1-2 g of acryloyl chloride and slowly add i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com