Method for preparing fine calcium phosphate by weak acid leaching of triple super phosphate
A calcium phosphate, leaching technology, applied in phosphorous acid, phosphorus oxyacid and other directions, can solve the problems of small gypsum crystals, low liquid-solid ratio, large floor space, etc., to reduce filtration pressure, ensure product quality, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
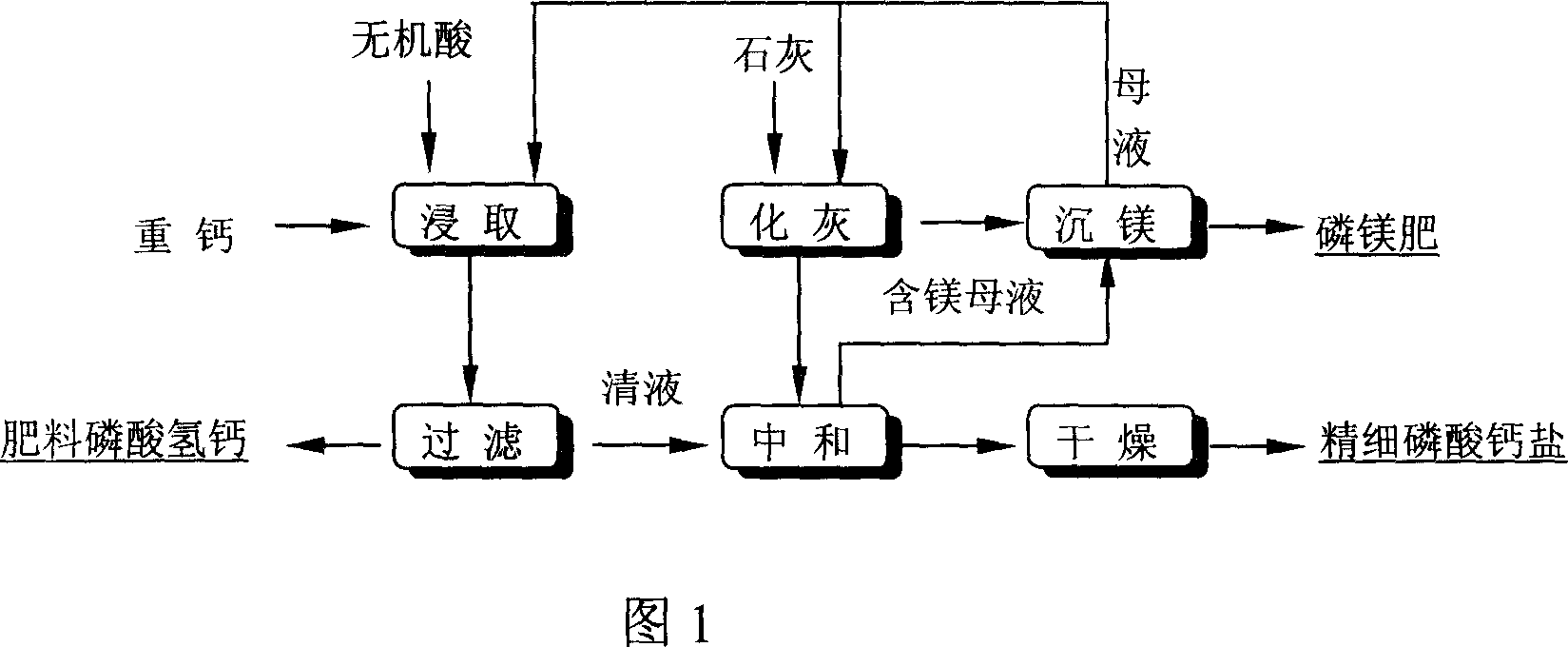
[0030] Weigh 2000g of heavy superphosphate (including total P 2 o 5 %44.6, Effective P 2 o 5 % 42.9, Free P 2 o 5 % 4.2, MgO% 1.42), and 16775g water slurry, leaching at 50 ℃, leaching time is 2 hours. After that, wet-process phosphoric acid (P 2 o 5 20%) to adjust the pH, share dephosphoric acid 425g, control the pH end point to be: pH=2.65, filter, filter residue (fertilizer grade calcium hydrogen phosphate) weight: 234.5g (containing P 2 o 5 29.1%); filtrate continues to be neutralized with milk of lime, and neutralization temperature is:~45 ℃, and the time is 1.5 hours, and when pH=6.5, filter and dry promptly obtain feed grade calcium hydrogen phosphate 2281g (38.7%P 2 o 5 ), the total yield of phosphorus pentoxide is: 90.35%.
Embodiment 2
[0032] According to the method for example 1, with the raw material of example 1, take double superphosphate 5000g and 25000g water slurry, after leaching, the phosphoric acid of humidification method is regulated pH, and the control terminal pH is 2.8, shares phosphoric acid (P 2 o 5 20%) 450g. Filtered, Fertilizer Grade Calcium Hydrogen Phosphate (with P 2 o 5 31.2%) weight 2684g, obtain product 3724g (containing P 2 o 5 39%), the total yield of phosphorus pentoxide is: 62.6%.
Embodiment 3
[0034] Weigh 2000g of heavy superphosphate (including total P 2 o 5 %44.3, Effective P 2 o 5 % 42.5, Free P 2 o 5 %4.8, MgO%3.21), mixed with 16775g water, leached at 50°C, and the leaching time was 2 hours. Afterwards, adjust pH with nitric acid (65%), share denitrification 110g, control pH end point is: pH=2.7, filter, filter residue (fertilizer grade calcium hydrogen phosphate) weight: 315.2g (containing P 2 o 5 30.3%); Filtrate continues to be neutralized with milk of lime, and neutralization temperature is:~45 ℃, and the time is 1.5 hours, and when pH=6.5, filter and dry promptly obtain feed grade calcium hydrogen phosphate 2012g (38.9%P 2 o 5 ), the total yield of phosphorus pentoxide is: 88.33%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com