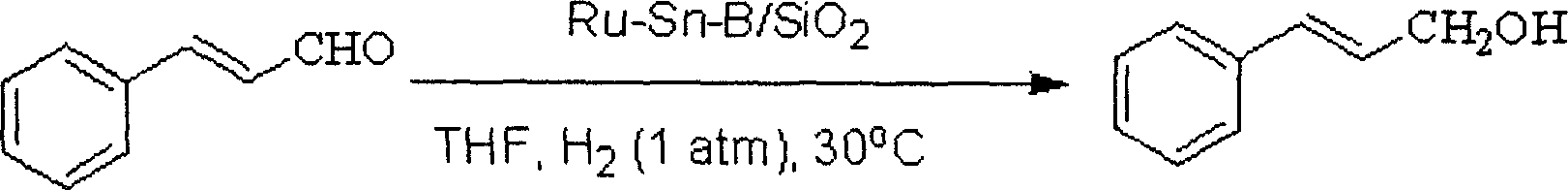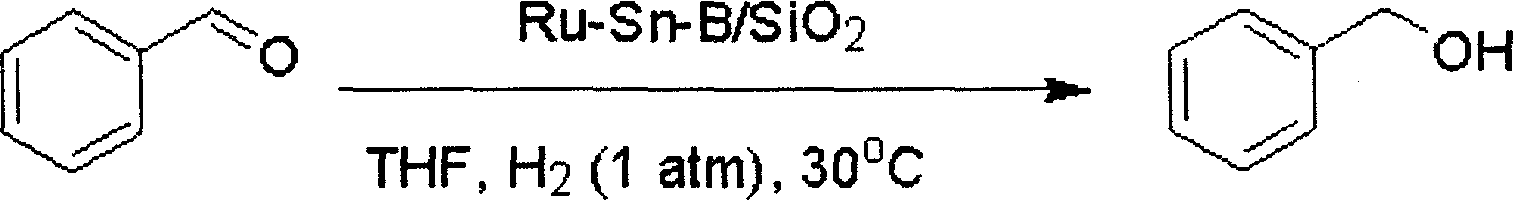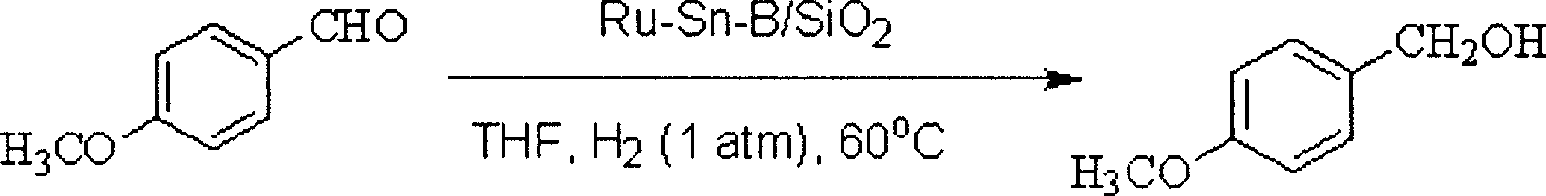Method for load type amorphous ruthenium boron-containing catalyst to catalyzing hydrogenating and reducing carbonyl compound to prepare alcohol
A carbonyl compound and catalytic hydrogenation technology, which is applied in the preparation of hydroxyl compounds, the preparation of organic compounds, catalyst carriers, etc., can solve the problems of high price of ruthenium metal, and achieve the effects of simple reaction operation, no pollution cost, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The reduction reaction of cinnamaldehyde is carried out in a glass tube, and the reaction tube is heated by an oil bath. 0.2g of Ru-Sn-B / SiO 2 Put the catalyst into a glass reaction tube, feed 0.1MPa hydrogen, pre-reduce at 180°C for 2 hours, control the reaction temperature at 30°C, add 7mmol cinnamaldehyde and 5mL tetrahydrofuran, and mix thoroughly in the reaction tube, provided by Shimadzu Corporation GC-14C type gas chromatography was used to detect the degree of reaction, and naphthalene was used as an internal standard. The reaction formula is as follows:
[0024]
[0025] After reacting for 5 hours under the above conditions, the conversion rate of cinnamaldehyde and the selectivity of cinnamyl alcohol both reached 100%.
Embodiment 2
[0027] The reduction reaction of benzaldehyde is carried out in a glass tube, and the reaction tube is heated by an oil bath. 0.2g of Ru-Sn-B / SiO 2 The catalyst was added into a glass reaction tube, and 0.1 MPa of hydrogen gas was introduced. After pre-reduction at 180°C for 2 hours, the reaction temperature was controlled at 30°C, 7mmol of benzaldehyde and 5mL of tetrahydrofuran were added, and mixed thoroughly in the reaction tube, provided by Shimadzu Corporation GC-14C type gas chromatography was used to detect the degree of reaction, and naphthalene was used as an internal standard. The reaction formula is as follows:
[0028]
[0029] After reacting for 12 hours under the above conditions, the conversion rate of benzaldehyde and the selectivity of benzyl alcohol both reached 100%.
Embodiment 3
[0031] The reduction reaction of benzaldehyde is carried out in a glass tube, and the reaction tube is heated by an oil bath. 0.1g of Ru-Sn-B / SiO 2 The catalyst was added to the glass reaction tube, 0.1MPa hydrogen gas was passed through, and after pre-reduction at 180°C for 2 hours, the reaction temperature was controlled at 70°C, 7mmol of benzaldehyde and 5mL of ethanol were added, and mixed thoroughly in the reaction tube, provided by Shimadzu Corporation GC-14C type gas chromatography was used to detect the degree of reaction, and naphthalene was used as an internal standard. After 14 hours of reaction, the conversion rate of benzaldehyde and the selectivity of benzyl alcohol both reached 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com