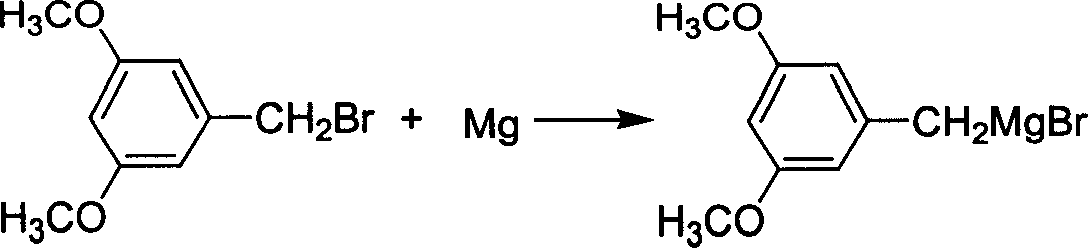Method for synthesizing resveratrol
A technology of resveratrol and trimethoxydiphenylethanol, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the harsh conditions of the reaction, the difficulty of industrialization, and the low yield of the reaction. problems, to achieve the effect of moderate reaction conditions, a wide range of solvents, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: prepare 3,5-dimethoxybenzylmagnesium bromide (being Grignard reagent) by 3,5-dimethoxybenzyl bromide
[0038] Add 26g (1.07mol) of polished magnesium chips and 100mL of anhydrous THF into a dry 1000mL three-necked reaction flask, start stirring, and heat to 60°C under nitrogen protection; slowly add 231g (1mol) of 3,5-dimethoxy The solution of benzyl bromide and 400mL THF was heated to reflux and stirred for 1h, then cooled; reacted in ultrasonic wave for 10min, the reaction was violent, and after the magnesium strip disappeared, it was cooled for later use.
Embodiment 2
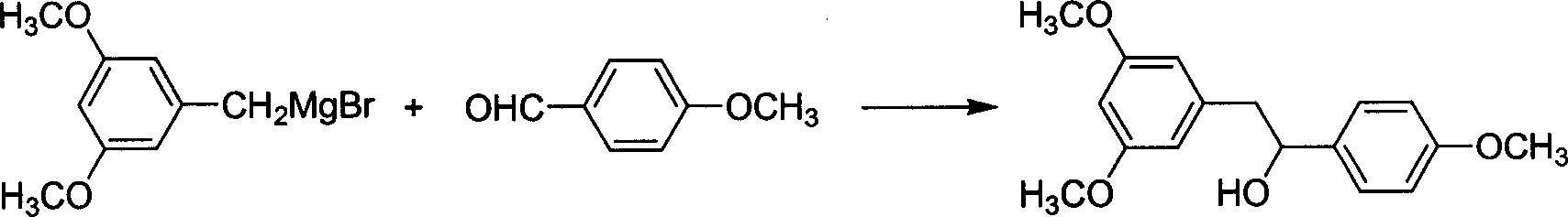
[0039] Embodiment 2: Prepare 3,5,4'-trimethoxybenzidine by 3,5-dimethoxybenzylmagnesium bromide
[0040] In a dry 2000mL three-necked reaction flask, add 140g (1.03mol) of anisaldehyde and 40mL of anhydrous THF, cool in an ice-water bath to below 10°C; start stirring, and slowly add the 3,5-dimethoxybenzyl prepared in Example 1 Magnesium bromide THF solution, keep the temperature fluctuating within 10-15°C; after the dropwise addition, stir at room temperature for 2h. After the reaction is complete, cool in an ice bath, add ammonium chloride (30 g) and water (100 mL), and stir for 30 min; pour the reactant into 500 mL of water, and recover the solvent THF. Extracted with ethyl acetate (200 mL×3), dried with anhydrous calcium chloride, and rotary evaporated to obtain 245 g of white solid (yield 85%).
Embodiment 3
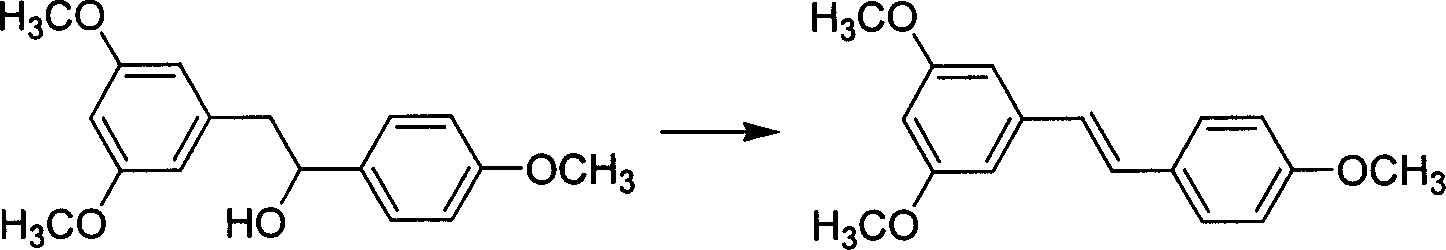
[0041] Embodiment 3: prepare 3,5,4'-trimethoxystilbene by 3,5,4,-trimethoxystilbene
[0042] Add 144g (0.5mol) of 3,5,4'-trimethoxybenzphenylethanol, 5g p-toluenesulfonic acid and 600mL anhydrous toluene into a dry 1000mL single-port reaction flask; install a reflux dehydration device; start stirring, and slowly Slowly heated to reflux; Reflux 18h. After the reaction is completed, the solvent toluene is recovered. The obtained solid was recrystallized in 95% ethanol to obtain 102 g of off-white solid (yield 76%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com