Long-lasting recombinant human red blood cell growth factor fusion protein and method
A growth factor and fusion protein technology, which is applied in the field of long-acting recombinant human erythrocyte growth factor fusion protein and its preparation, and can solve the problems of low activity and reducing the number of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1 The connecting fragment used in this example is a connecting peptide, the primary structure of the connecting peptide sequence, that is, the protein sequence connecting the dimer / trimer, is a connecting sequence of 9-20 amino acids, with these connecting sequences All amino acids can be used, and a sequence shown in Figure 7 is used in this example.
[0078] Design and preparation of primer oligodeoxyribonucleic acid:
[0079] P1: 5'TGGGGGTGCACGAATGTCCTGC 3'
[0080] P1 starts from the EPO gene initiation factor, including the signal peptide chain.
[0081] P2: 5'TCATCTGTCCCCTGTCCTGCAGG3'
[0082] P2 is an oligodeoxyribonucleic acid complementary to the 3' end, together with P1, by PCR,
[0083] Amplification and cloning of full-length (protein coding) EPO cDNA. The subsequent subcloning and duplex / trimer construction will be based on this plasmid.
[0084] P3: 5'AAGCTAGGATCCATGGGGGTGCACGAATGTCCTGC 3'
[0085] The latter part of P3 is consistent with the...
Embodiment 2
[0093] Example 2 Screening and determination of LINKER sequences used in EPO-EPO and EPO-EPO-EPO:
[0094] After a series of pre-tests and tests, the following connecting peptide sequences were determined, including but not limited to the following sequences:
[0095] Gly-Gly-Ser-Gly-Ala-Ala-Ser-Gly-Gly;
[0096] Gly-Gly-Ser-Gly-Ala-Ala-Ser-Gly-Ser-Ser;
[0097] Gly-Gly-Gly-Gly-Ala-Ala-Ser-Gly-Ser-Ser-Ala;
[0098] Gly-Gly-Gly-Gly-Ala-Ala-Ser-Gly-Ser-Ser-Ala-Gly;
[0099] Gly-Gly-Ser-Gly-Gly-Gly-Ser-Ala-Ala-Gly-Gly-Ser-Gly;
[0100] Gly-Gly-Ser-Gly-Gly-Gly-Ser-Ala-Ala-Gly-Gly-Ser-Gly-Gly;
[0101] Gly-Gly-Gly-Gly-Ala-Ala-Ser-Gly-Ser-Ser-Ala-Gly-Ser-Ala-Ala;
[0102] Gly-Gly-Ser-Gly-Ala-Ala-Ser-Gly-Ser-Ser-Ala-Gly-Gly-Ser-Gly-Gly;
[0103] Gly-Gly-Ser-Gly-Gly-Gly-Ser-Ala-Ala-Gly-Gly-Ser-Gly-Gly-Ser-Ala-Ala;
[0104] Gly-Gly-Ser-Gly-Gly-Gly-Ser-Ala-Ala-Gly-Gly-Ser-Gly-Gly-Ser-Gly-Ser-Ser-Ala-Gly;
[0105] Gly-Gly-Scr-Gly-Gly-Gly-Ser-Ala-Ala-Gly-Gly-Ser-Gly-Gly-Ser-Gly-Se...
Embodiment 3
[0108] Embodiment 3 EPO, the determination of the full-length nucleotide sequence of EPO-EPO and EPO-EPO-EPO fusion gene:
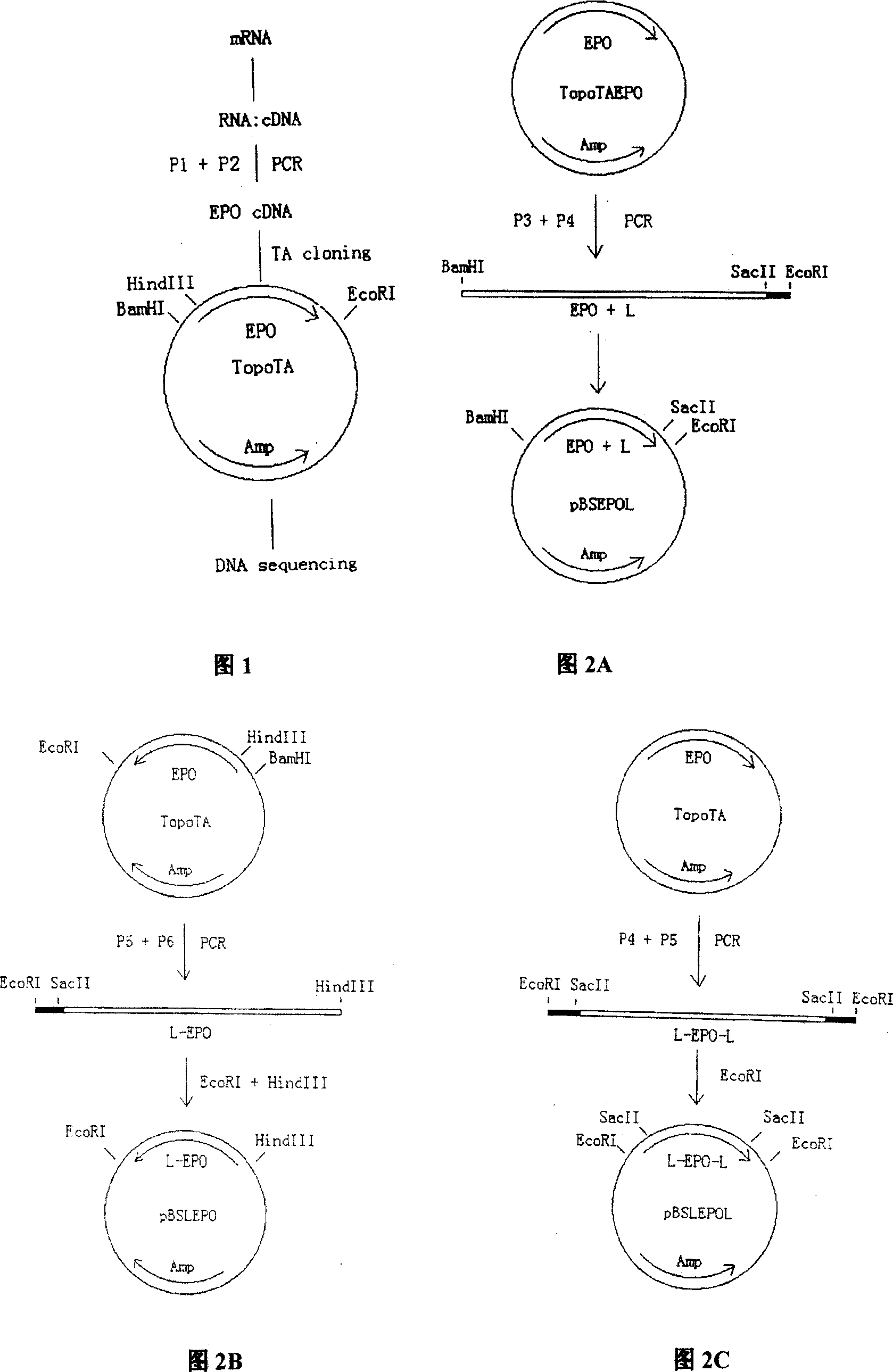
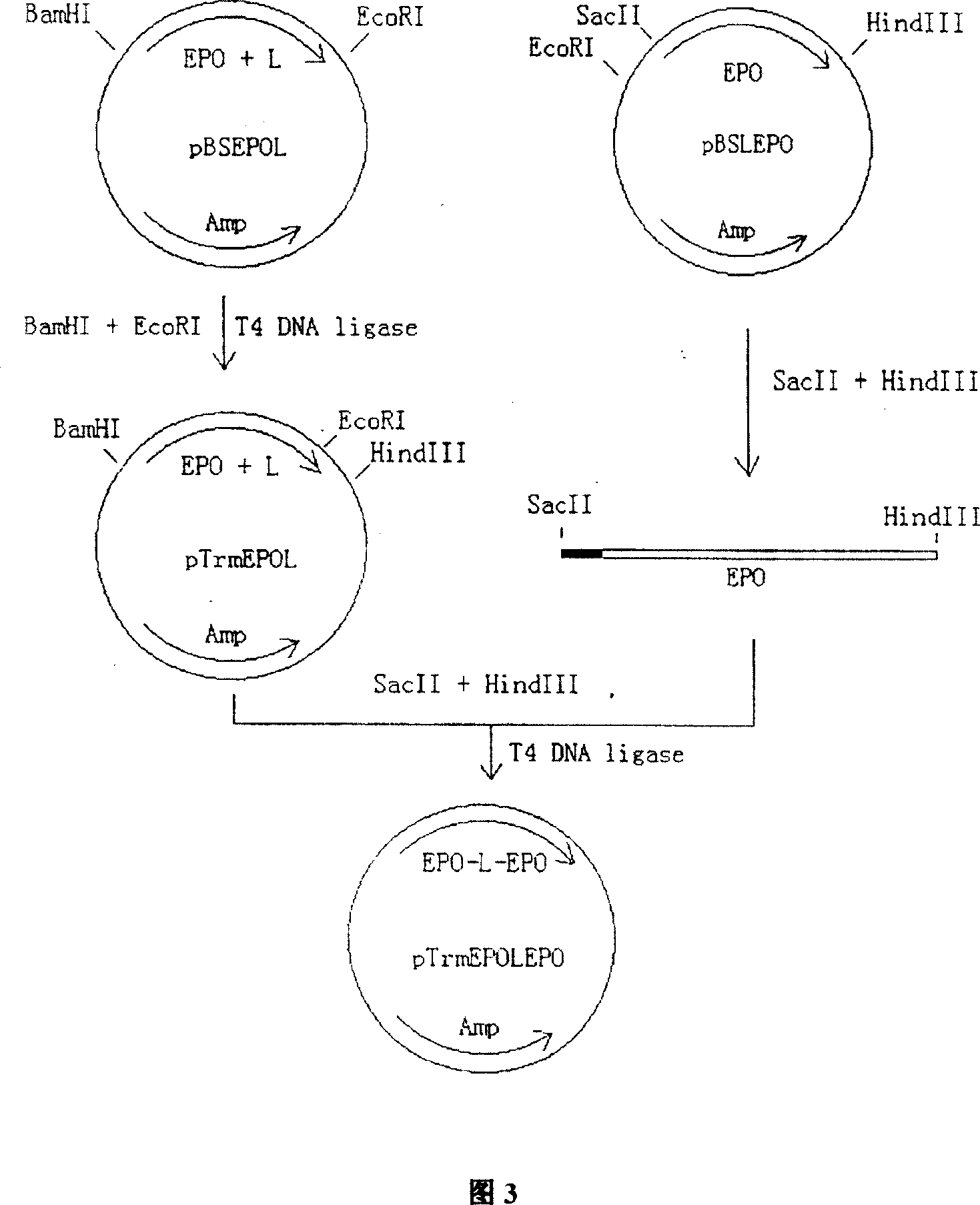
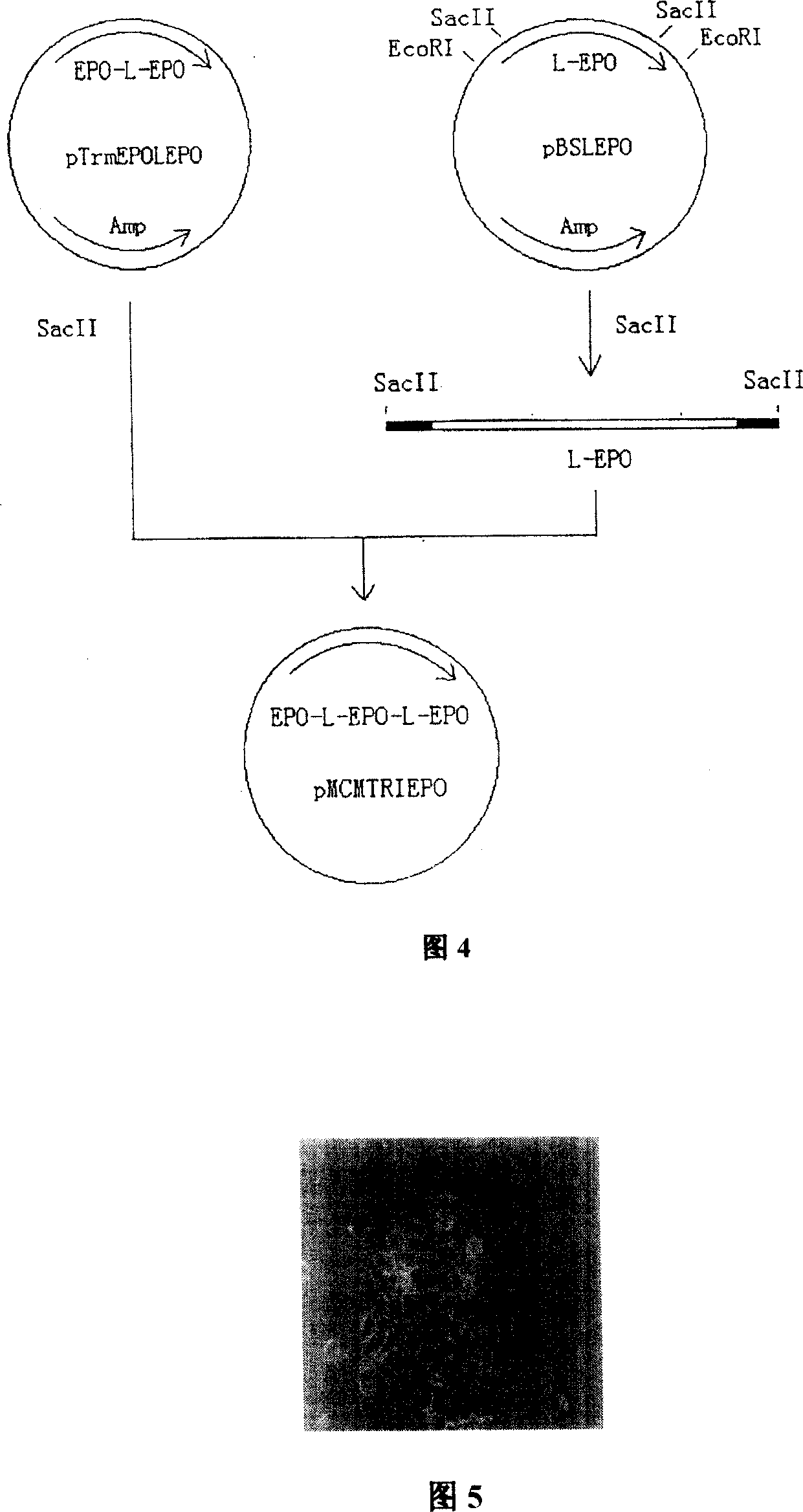
[0109] In order to obtain the EPO gene, EPO-EPO and EPO-EPO-EPO fusion genes, the cDNA and the modified sequences were subcloned and terminally modified, and the nucleotides of each cDNA and the modified fusion genes were carried out by conventional methods. Sequence determination (see Example 1, Figure 2, Figure 3, Figure 4 for details).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com