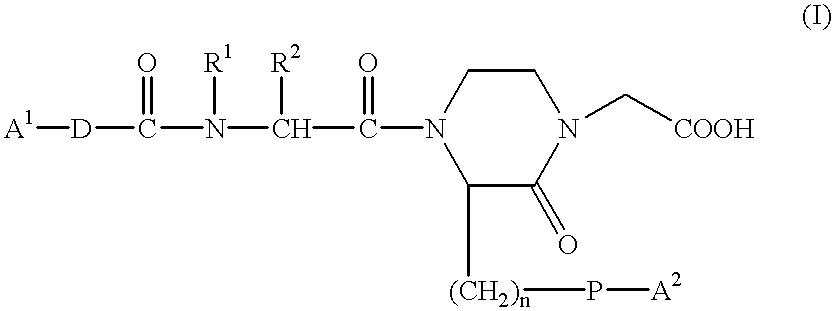
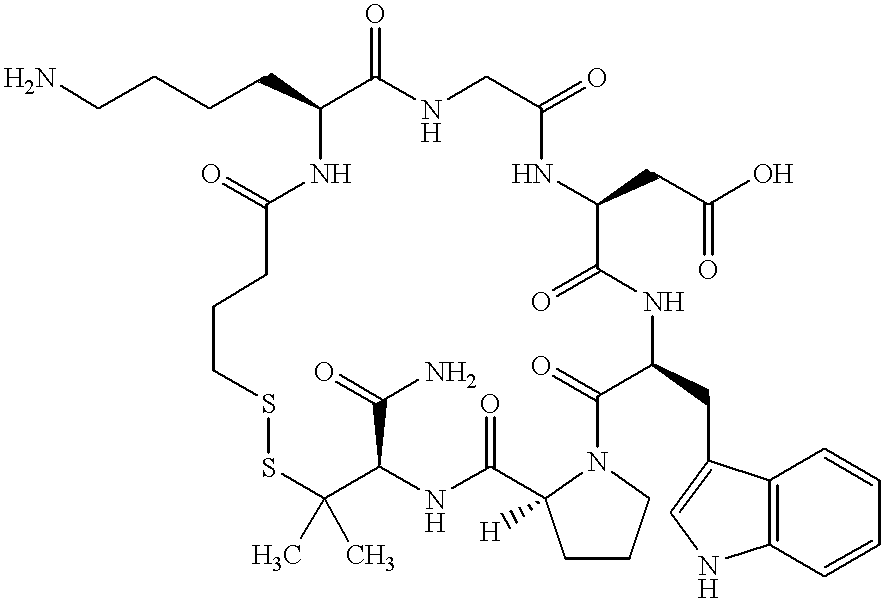
Method for transdermal administration of gp iib/iiia antagonist
a technology of gp iib and iiia, which is applied in the field of transdermal administration of gp iib/iiia antagonist, can solve the problems of side effects, prolonging bleeding time, and not showing therapeutic efficacy without maintaining a long-term pharmacologically effective serum concentration, so as to reduce the risk of side effects, maintain stable, and excellent pharmacological
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
[0245] As the device for transdermal administration by iontophoresis, a device comprising of an anode patch and a cathode patch was prepared.
[0246] As illustrated in FIG. 2, the anode patch comprises a foil silver electrode 1, a conductive layer 2 comprising 1.0%(w / w) of an agar gel containing the about 5%(w / w) ion exchange resin cholestyramine, additives (5%(w / w) of urea, 10%(w / w) of proline, 0.1%(w / w) sodium benzoate, 0.03 t(w / w) citric acid) and a thickener(0.25%(w / w) of xanthan gum and 0.25%(w / w) of locust bean gum), a cup-shaped support 3 (inner diameter: about 30 mm, thickness: about 1.5 mm, volume: about 1.3 mL, weight: about 1.3 g) for holding 1 and 2, an adhesive member 4 for affixing the device in position on the skin, and a porous membrane (drug-holding membrane) 5 for holding the drug [Hydrophilic Durapore (TM), Nippon Millipore]. The area of contact surface of the anode patch with the skin is about 9 cm.sup.2.
[0247] The cathode patch was comprising a print silver / silver...
reference example 2
[0249] Except that 609 .mu.g of the compound synthesized in Production Example was applied to the drug-holding membrane, the transdermal administration of the compound by iontophoresis and serial determination of its serum concentration were carried out in otherwise the same manner as in Reference Example 1.
reference example 3
[0250] Except that 2494 .mu.g of the compound synthesized in Production Example 1 was applied to the drug-holding membrane, the transdermal administration of the compound by iontophoresis and serial determination of its serum concentration were carried out in otherwise the same manner as in Reference Example 1.
[0251] The time course of the serum concentration of the compound synthesized in Production Example 1 following the commencement of administration in each of Reference Examples 1 to 3 is presented in FIG. 3. The results indicated that current-responsive blood concentrations were obtained. As shown in FIG. 3, the compound was absorbed through the skin in relation to the electrical current applied, resulting in an increasing serum concentration of the compound. Furthermore, the absorption by the iontophoresis of the compound was dose-dependent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com