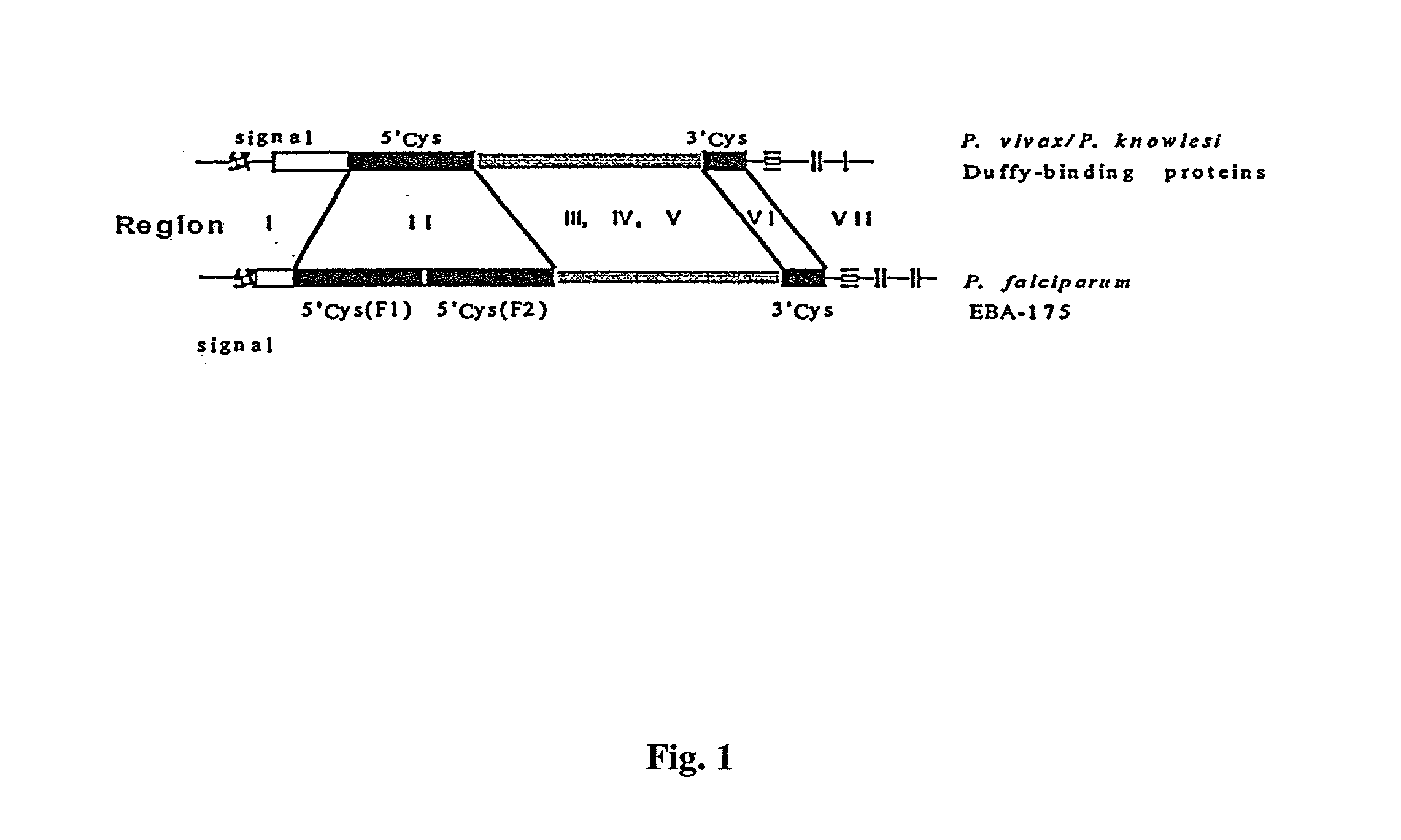
Anti-plasmodium compositions and methods of use
a technology of compositions and anti-plasmodium, applied in the field of microbiology and immunology, can solve problems such as coma, rapid death, and convulsions, and achieve the effects of preventing coma, rapid death, and preventing coma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods for Detecting P. falciparum EBPs
[0098] Genomic Database
[0099] The sequence data for P. falciparum chromosome 13 was obtained from The Sanger Centre website at http: / / www.sanger.ac.uk / Projects / P_falciparu- m / . Sequencing of P. falciparum chromosome 13 was accomplished as part of the Malaria Genome Project with support by The Wellcome Trust.
[0100] Parasites
[0101] Plasmodium falciparum 3D7 strain (clone of NF54, Amsterdam Airport, human challenge strain) and FVO strain (Aotus adapted) were maintained as previously reported (Vemes et al., 1984). When appropriate, schizonts were purified on Percoll density gradient. The 3D7 parasites were metabolically labeled with TRAN.sup.35S-LABEL.TM. (ICN Radiochemicals, Irvine, Calif.) as previously described (Sim et al., 1994b). Essentially 2.times.10.sup.8 parasites in 10 ml RPMI-1640 culture media deficient in methionine and cysteine were incubated with 1 mCi TRAN.sup.35S-LABEL.TM. for 4 hours for parasite-cell pellets and 1...
example 2
Expression of EBP2 in Parasitized Erythrocytes
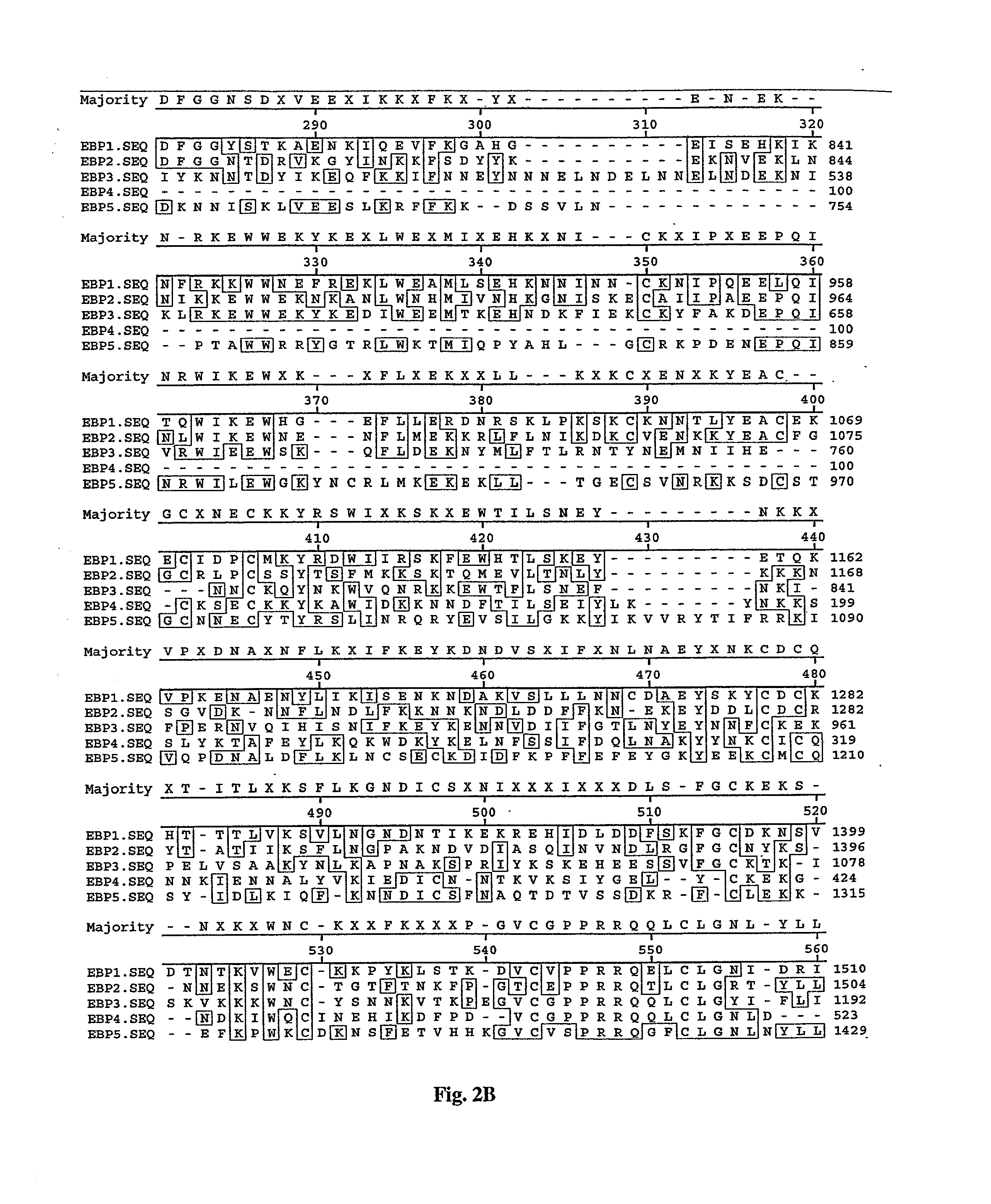
[0115] To examine whether ebp2 was transcribed a mRNA transcript was detected with RT-PCR. Parasite mRNA isolated from 3D7 schizont-infected erythrocytes was used for first strand synthesis with random primers or with a poly dT primer and then PCR amplified with an ebp2 primer pair or eba-175 primer pair as a control. Appropriate size DNA fragments were detected on an ethidium bromide stained agarose gel for ebp2 and eba-175. Using genomic 3D7 DNA, ebp2 RII was amplified by PCR and cloned into a naked DNA vaccine plasmid (Hartikka et al., 1996). Both the forward and reverse strands of the DNA fragment were sequenced. A single point nucleotide change at position 1654 (A to G) encoded an amino acid substitution from Asn to Asp. This single amino acid difference is the result of a PCR-introduced artifact.
[0116] Recombinant EBP2 RII and EBA-175 RII derived from supernatants by transient transfection of UM449 cells were immunoblotted with spe...
example 3
EBP2 RII Specific Antibodies Recognize a 130 kDa P. falciparum Protein
[0119] To determine the molecular mass of EBP2 [.sup.35S]-metabolically labeled P. falciparum 3D7 strain schizont-infected erythrocyte lysates were incubated with EBP2 RII specific antibodies coupled to Protein G-sepharose. [.sup.35S]-labeled P. falciparum schizont-infected erythrocyte lysate were immunoprecipitated and human erythrocyte bound [.sup.35S]-labeled EBP2 were detected from [.sup.35S]-labeled parasite culture supernatants. EBA-175 specific polyclonal rabbit IgG was included as a control. Results were obtained with EBP2 specific polyclonal sera or control and EBA-175 specific polyclonal IgG and control. [.sup.35S]-labeled EBP2 and EBA-175 were immunoprecipitated from lysates of erythrocytes with bound EBPs. [.sup.35S]-labeled EBP2 and EBA-175 were eluted off human erythrocytes with 1M NaCl and then immunoprecipitated with EBP2 or EBA-175 specific antibodies. Mouse and rabbit adjuvant controls and molecu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com