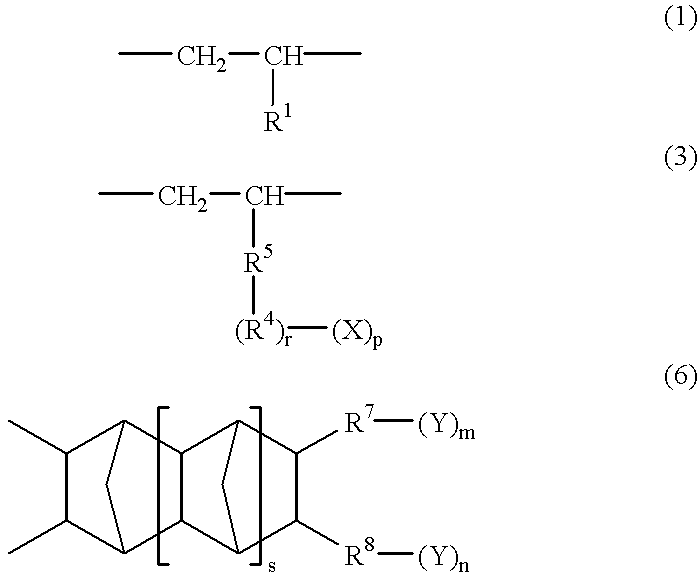
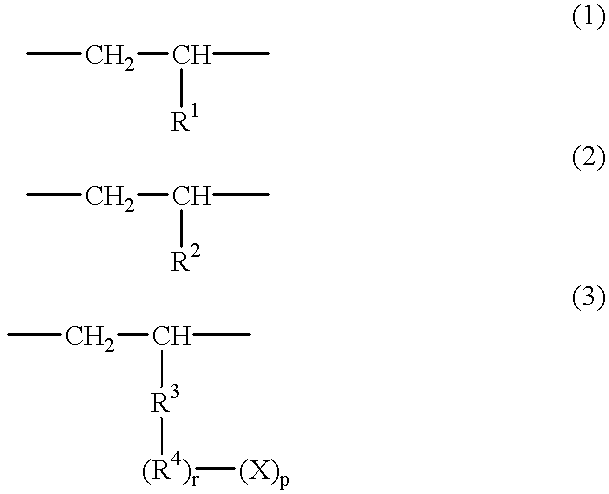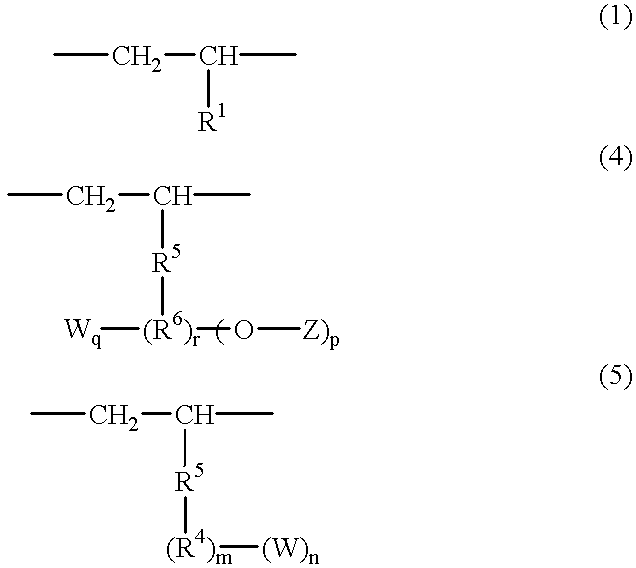Polar group-containing olefin copolymer, process for preparing the same, thermoplastic resin composition containing the copolymer, and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[1574] In a 1000 ml glass polymerization reactor thoroughly purged with nitrogen, 400 ml of n-decane was placed, then nitrogen was passed through at a rate of 20 l / hr, and the contents were maintained at 130.degree. C. for 10 minutes. Then, 0.6 mmol of triisobutylaluminum was added, followed by further adding 0.48 mmol of undecen-1-ol (having been dried over activated alumina) represented by the following formula. 192
[1575] Then, 1.100 mmol of methylaluminoxane was further added, and passing of nitrogen was stopped, followed by passing ethylene at a rate of 12.5 l / hr. Finally, a toluene slurry solution in which 0.002 mmol of dimethylsilylene(2,7-dimethyl-4,5-(2-methyl-benzo)-1-indenyl)(2,7-di-tert--butylfluorenyl)zirconium dichloride and 0.500 mmol of methylaluminoxane had been contacted at room temperature for 10 minutes was added to initiate polymerization. After the polymerization was conducted at 130.degree. C. for 1 hour at atmospheric pressure, a small amount of isobutyl alcoh...
example 2
[1578] In a 1000 ml glass polymerization reactor thoroughly purged with nitrogen, 400 ml of toluene was placed, then nitrogen was passed through at a rate of 20 l / hr, and the contents were maintained at 90.degree. C. for 10 minutes. Then, 0.6 mmol of triisobutylaluminum was added, followed by further adding 0.48 mmol of 1,2-epoxy-9-decene (having been dried over silica alumina) represented by the following formula. 193
[1579] Then, 1.100 mmol of methylaluminoxane was further added, and passing of nitrogen was stopped, followed by passing ethylene at a rate of 12.5 l / hr. Finally, a toluene slurry solution in which 0.002 mmol of dimethylsilylene(2,7-dimethyl-4,5-(2-methyl-benzo)-1-indenyl)(2,7-di-tert--butylfluorenyl)zirconium dichloride and 0.500 mmol of methylaluminoxane had been contacted at room temperature for 10 minutes was added to initiate polymerization. After the polymerization was conducted at 90.degree. C. for 1 hour at atmospheric pressure, a small amount of isobutyl alcoh...
example 3
[1582] In a 1000 ml glass polymerization reactor thoroughly purged with nitrogen, 400 ml of n-decane was placed, then nitrogen was passed through at a rate of 20 l / hr, and the contents were maintained at 90.degree. C. for 10 minutes. Then, 0.6 mmol of triisobutylaluminum was added, followed by further adding 0.48 mmol of (2,7-octadien-1-yl)succinic anhydride (having been dried over activated alumina) represented by the following formula. 194
[1583] Then, 1.100 mmol of methylaluminoxane was further added, and passing of nitrogen was stopped, followed by passing ethylene at a rate of 12.5 l / hr. Finally, a toluene slurry solution in which 0.002 mmol of dimethylsilylene(2,7-dimethyl-4,5-(2-methyl-benzo)-1-indenyl)(2,7-di-tert--butylfluorenyl)zirconium dichloride and 0.500 mmol of methylaluminoxane had been contacted at room temperature for 10 minutes was added to initiate polymerization. After the polymerization was conducted at 130.degree. C. for 1 hour at atmospheric pressure, a small ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com