Polymers containing functionalized carbon nanotubes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
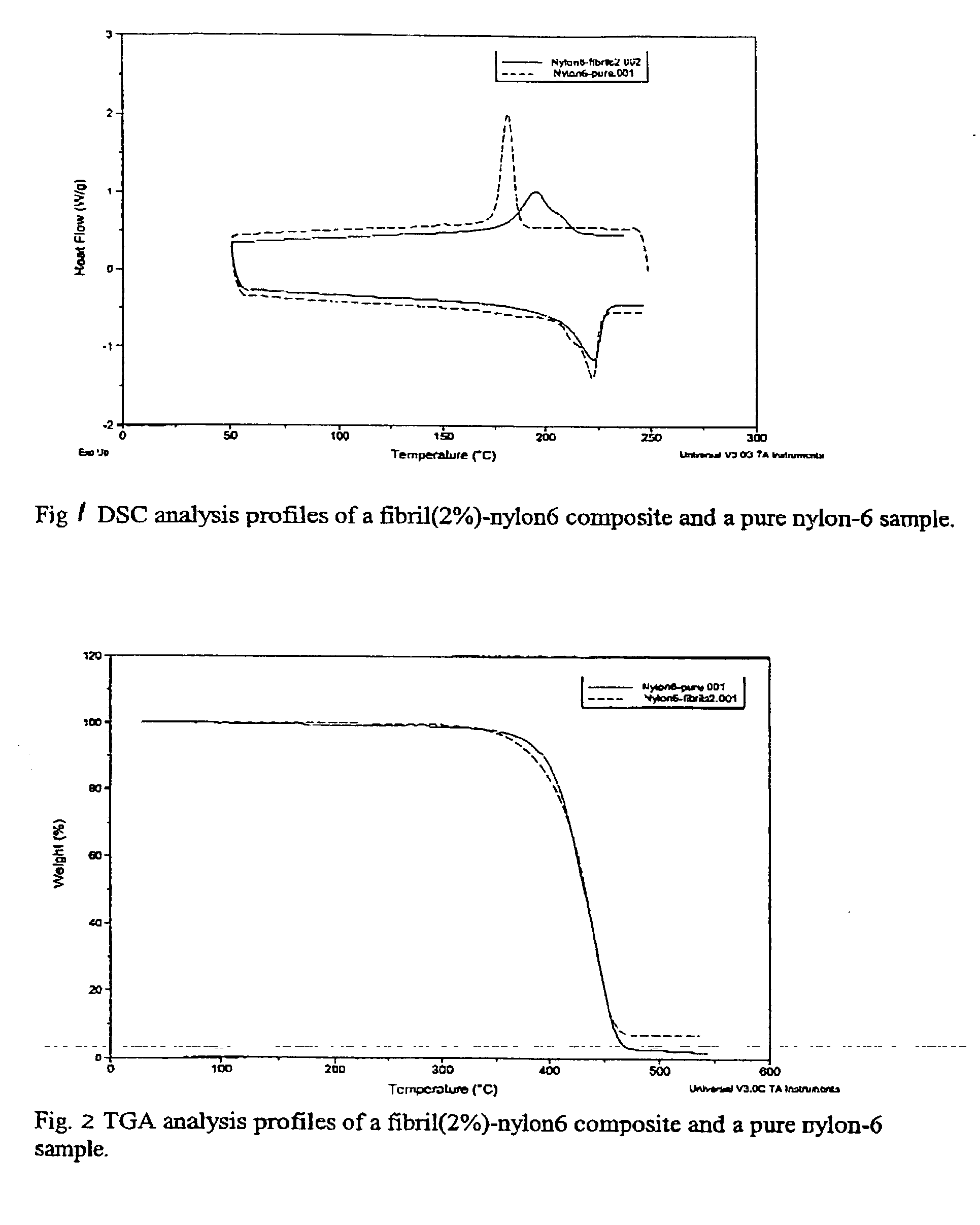
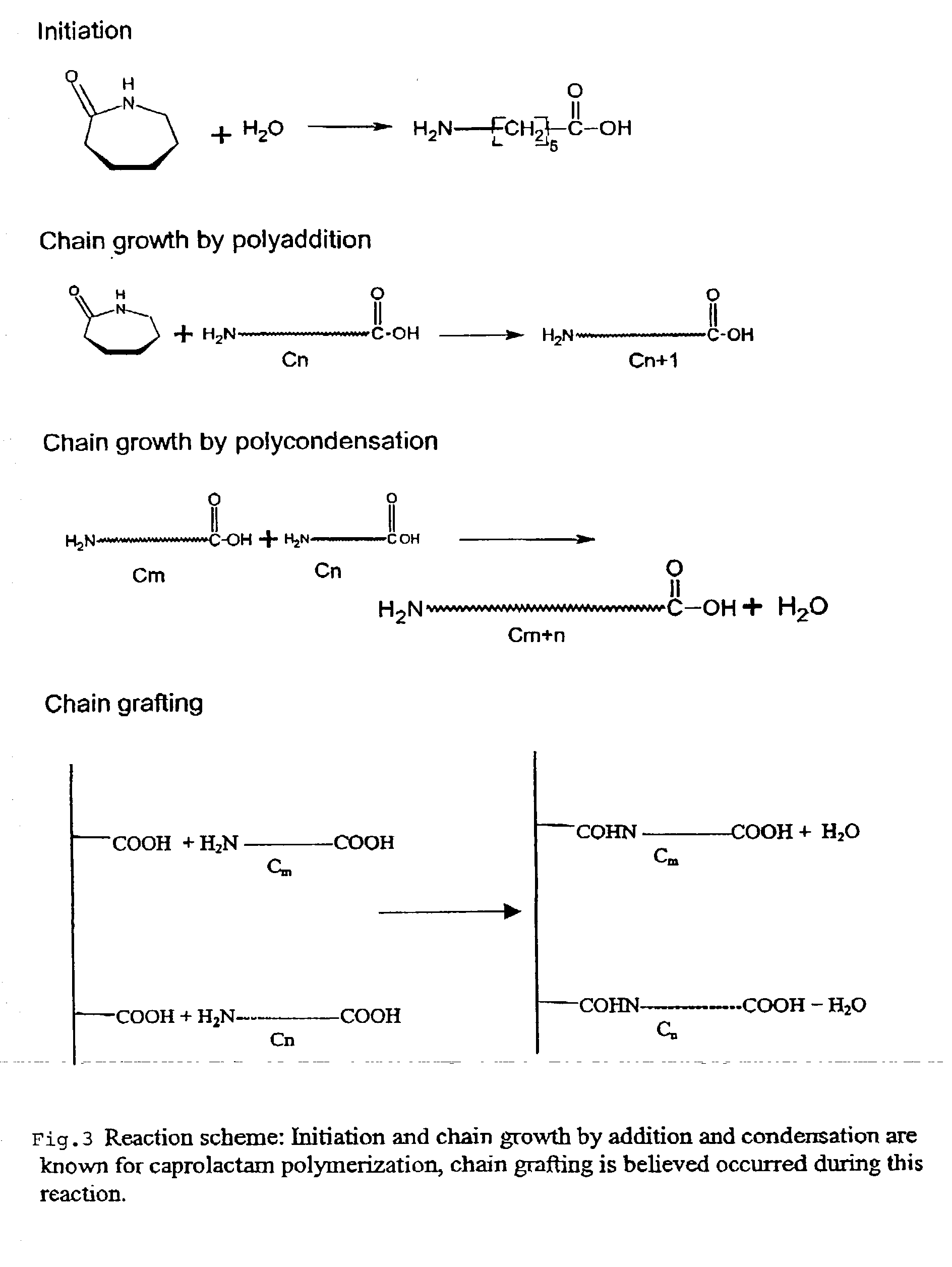
Image
Examples
example 1
Preparation of Carboxylic Acid-Functionalized Fibrils Using Chlorate
[0095] A sample of CC carbon nanotubes was slurried in concentrated H.sub.2SO.sub.4 by mixing with a spatula and then transferred to a reactor flask fitted with gas inlet / outlets and an overhead stirrer. With stirring and under a slow flow of argon, the charge of NaClO.sub.3 was added in portions at room temperature over the duration of the run. Chlorine vapors were generated during the entire course of the run and were swept out of the reactor into a aqueous NaOH trap. At the end of the run, the fibril slurry was poured over cracked ice and vacuum filtered. The filter cake was then transferred to a Soxhlet thimble and washed in a Soxhlet extractor with deionized water, exchanging fresh water every several hours. Washing was continued until a sample of the carbon nanotubes, when added to fresh deionized water, did not change the pH of the water. The carbon nanotubes were then separated by filtration and dried at 100...
example 2
Preparation of Carboxylic Acid-Functionalized Fibrils Using Nitric Acid
[0096] A sample of carbon nanotubes was slurried with nitric acid of the appropriate strength in a bound bottom multi-neck indented reactor flask equipped with an overhead stirrer and a water condenser. With constant stirring, the temperature was adjusted and the reaction carried out for the specified time. Brown fumes were liberated shortly after the temperature exceeded 70.degree. C., regardless of acid strength. After the reaction, the slurry was poured onto cracked ice and diluted with deionized water. The slurry was filtered and excess acid removed by washing in a Soxhlet extractor, replacing the reservoir with fresh deionized water every several hours, until a slurried sample gave no change in pH from deionized water. The carbon nanotubes were dried at 100.degree. C. at 5" vacuum overnight.
example 3
Preparation of Amino-Functionalized Fibrils Using Nitric Acid
[0097] To a cooled suspension (0.degree. C.) of fibrils (70 mg) in water (1.6 ml) and acetic acid (0.8 ml) was added nitric acid (0.4 ml) in a dropwise manner. The reaction mixture was stirred for 15 minutes at 0.degree. C. and stirred for further 1 hour at room temperature. A mixture of sulfuric acid (0.4 ml) and hydrochloric acid (0.4 ml) was added slowly and stirred for 1 hour at room temperature. The reaction was stopped and centrifuged. The aqueous layer was removed and the fibrils washed with water (X5). The residue was treated with 10% sodium hydroxide (X3), and washed with water (X5) to furnish nitrated fibrils.
[0098] To a suspension of nitrated fibrils in water (3 ml) and ammonium hydroxide (2 ml) was added sodium dithionite (200 mg) in three portions at 0.degree. C. The reaction mixture was stirred for 5 minutes at room temperature and refluxed for 1 hour at 100.degree. C. The reaction was stopped, cooled to 0.de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com