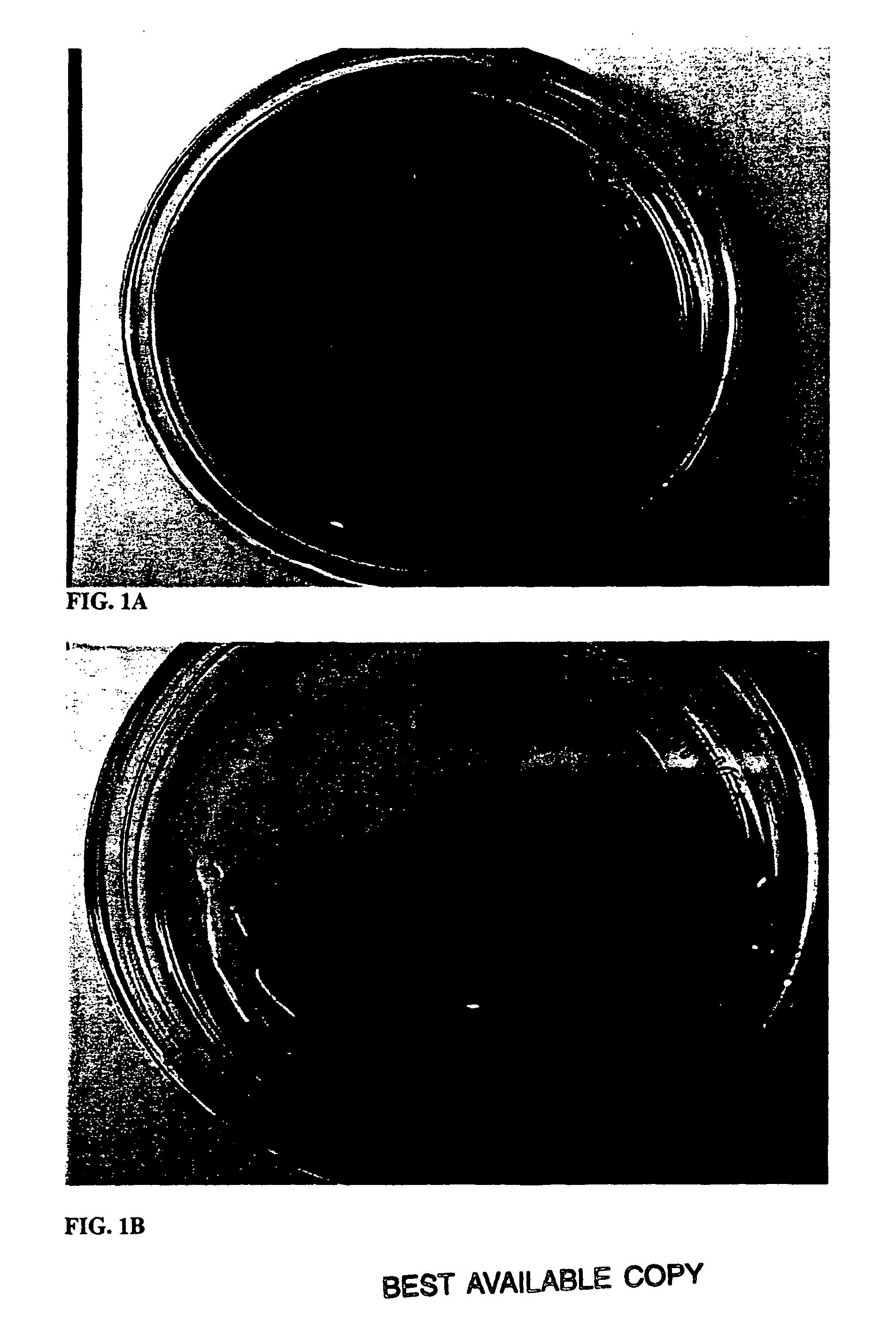
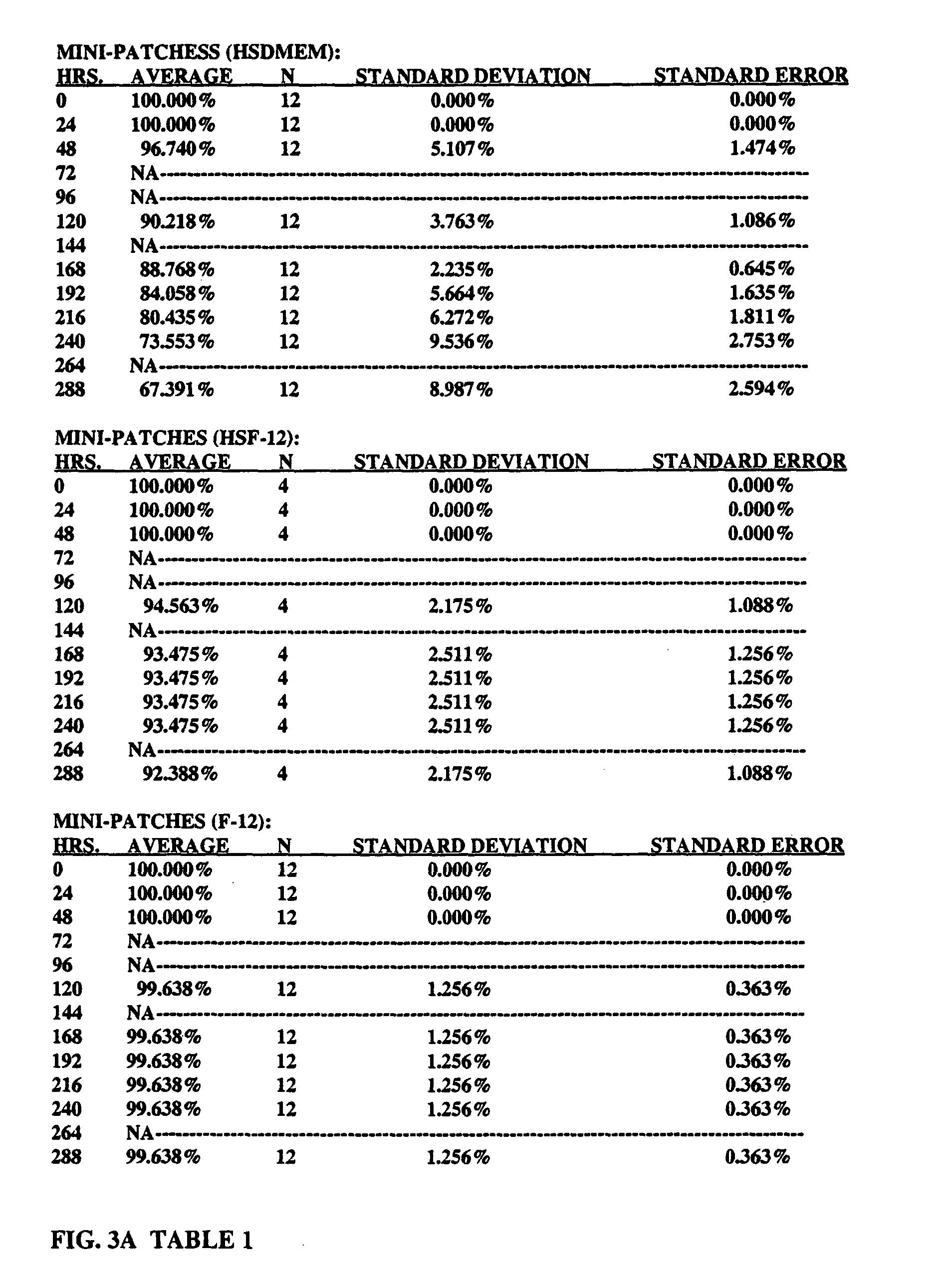
Pericardial anti-adhesion patch
a technology of anti-adhesion patch and pericardium, which is applied in the direction of prosthesis, biocide, bandages, etc., can solve the problems of insufficient hemostasis, prolonged contact with foreign materials, and failure to remove all abnormal tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0034] Sources of Cells
[0035] Normal human dermal fibroblasts may be purchased from Clonetics-Biowhitaker either as live cultures (in tissue culture flasks) or as ampoules of frozen stock cells stored under liquid nitrogen (N.sub.2). The frozen cells are allowed to thaw until the ice pellet is free to move in the vial. The contents of the vial are pipeted into a 75 cm.sup.2 vented cap tissue culture flask. To the flask is then added 15 ml Dulbeco's Modified Eagle Medium (DMEM) containing 10% FBS and the culture incubated at 37.degree. and 5% CO.sub.2 containing air. The medium is changed every second day until the cells populate about 80-90% of the growing. Fibroblasts are then further sub-cultured to expand numbers under the same conditions in DMEM containing 10% FBS. If the cells are purchased as cultures in 75 cm.sup.2 tissue culture flasks, the flasks do not have vented caps and are completely filled with medium with no air space. The transport medium is removed and fresh growth...
example 3
[0037] Harvesting Cells for Xenograft PAP
[0038] Skin tissue remnants from circumcisions are obtained from the local newborn nursery or maternity ward (OBGYN). These are first decontaminated by soaking the remnants in serum free DMEM containing 20% penicillin / streptomycin at 4.degree. C. The subcutaneous fat is then removed from the foreskin and the decontamination repeated using the same medium except the concentration of penicillin / streptomycin in DMEM is 10%. The skin sample is then incubated in dispase (10 unit / ml) for 48 hrs at 4.degree. C. after which time the epidermis can easily be pulled from, detached and removed from the dermis. The dermis is rinsed several times in PBS and then cut into very small pieces (2-3 mm.sup.2) which were placed on to the inner surface of a 75 cm.sup.2 vented cap tissue culture flask. The pieces were allowed to attach to the flask by incubating at 37.degree. C. in an incubator (5% CO.sub.2 and 98% humidity) for 10-20 min, after which time DMEM con...
example 4
[0039] Harvesting Cells for Allograft PAP
[0040] For this application the patient who decides on elective surgery has to donate a skin punch biopsy in order that his / her cells may be harvested and cultured to expand the cell numbers so that the cells could be incorporated into allograft PAP. The anatomical locations from which the punch biopsies are obtained are usually chosen to be sites that are not exposed and are esthetically acceptable to the patent. These are usually inside of the forearm or upper arm. The skin is cleaned, sterilized and local anesthetic administered. A full thickness 6-mm punch biopsy is then obtained and the wound closed with one or two stitches. The skin sample is kept sterile and is treated in the manner analogous to that described in Example 3 for the infant foreskin sample. Dermal fibroblasts are obtained in the identical fashion. When a sufficient cell number has been obtained, the PAP can be constructed and the preparation for surgery may begin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com