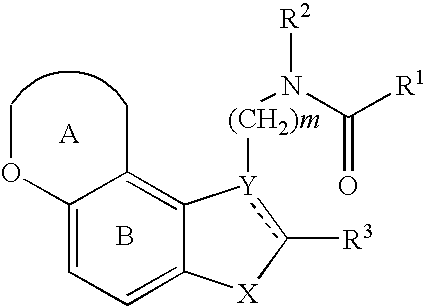
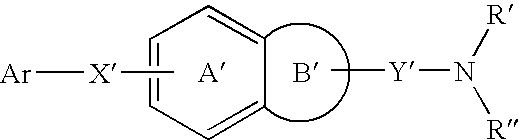
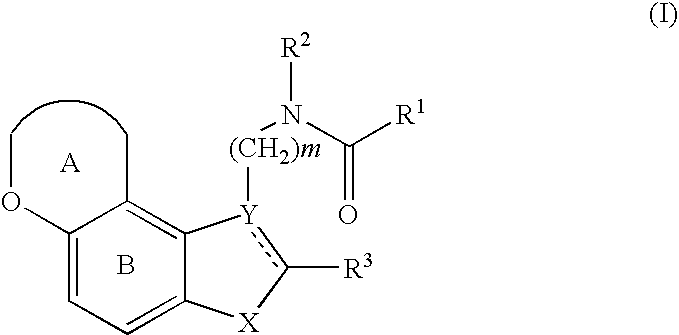
Pharmaceutical preparation containing copolyvidone
a technology of copolyvidone and pharmaceutical preparation, which is applied in the directions of organic chemistry, coatings, and active ingredients of heterocyclic compounds, can solve the problem of insufficient activity and achieve the effect of improving plasticity and film strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 2
Synthesis of Ethyl (E)-3-(2,3-Dihydrobenzofuran-5-yl)propenoate
[0336] To 2,3-dihydrobenzofuran-5-carbaldehyde obtained Reference Example 1 (832.3 mmol) / toluene solution (340 g), triethyl phosphonoacetate (205.3 g, 915.7 mmol) was added dropwise under cooling. Then, t-butoxy sodium (88.0 g, 1187.3 mmol) suspended in toluene (530 g) was added dropwise to the resultant mixture and, after stirring the mixture for one hour, acetic acid (20 g) and water (500 g) were further added dropwise thereto. The reaction mixture was raised to room temperature and partitioned. The organic layer was washed in turn with saturated sodium bicarbonate water and water and concentrated under reduced pressure until the volume is reduced to 300 mL or less. Methanol (396 g) was added and the mixture was heated to dissolve it. Water (500 g) was added dropwise thereto at room temperature and the mixture was stirred to deposit crystals. The crystals were collected by filtration and dried under reduced pressure to...
reference example 3
Synthesis of Ethyl 3-(2,3-Dihydrobenzofuran-5-yl)propionate
[0337] Ethyl (E)-3-(2,3-dihydrobenzofuran-5-yl)propionate (50.0 g, 227.3 mmol) was dissolved in acetic acid (312.0 g). After replacing the atmosphere in the system with nitrogen, 5% Pd / C (4.96 g) (as dry) was added to the solution and the system was pressurized with hydrogen up to 196 to 294 kPa. The reaction was conducted at 50.degree. C. under pressure of 196 to 294 kPa for one hour. The catalyst was removed by filtration, and then the reaction mixture was washed with acetic acid (208 g) to obtain an acetic acid solution of the title compound (yield: 569.3 g, apparent yield: 100%).
reference example 4
Synthesis of 3-(6,7-Dibromo-2,3-dihydrobenzofuran-5-yl)propionic Acid
[0338] To the PPE / acetic acid solution (569.3 g, 227.3 mmol) obtained from the process of Reference Example 3, anhydrous sodium acetate (18.6 g) was added. Bromine (221.6 g) was added dropwise to the resultant mixture over 2 hours under cooling with stirring. Then, the reaction was conducted at room temperature for 4 hours and the reaction mixture was added dropwise to a cooled aqueous 15% sodium sulfite solution (670 ml), followed by stirring for 30 minutes. Acetonitrile (118 g) was added thereto and the mixture was reacted by heating under reflux for 2 hours. The mixture was gradually cooled and stirred for one hour to deposit crystals. The crystals were collected by filtration, washed with water and then dried to obtain the title compound (yield: 63.3 g, 73.2%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com