Granular metallic iron
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
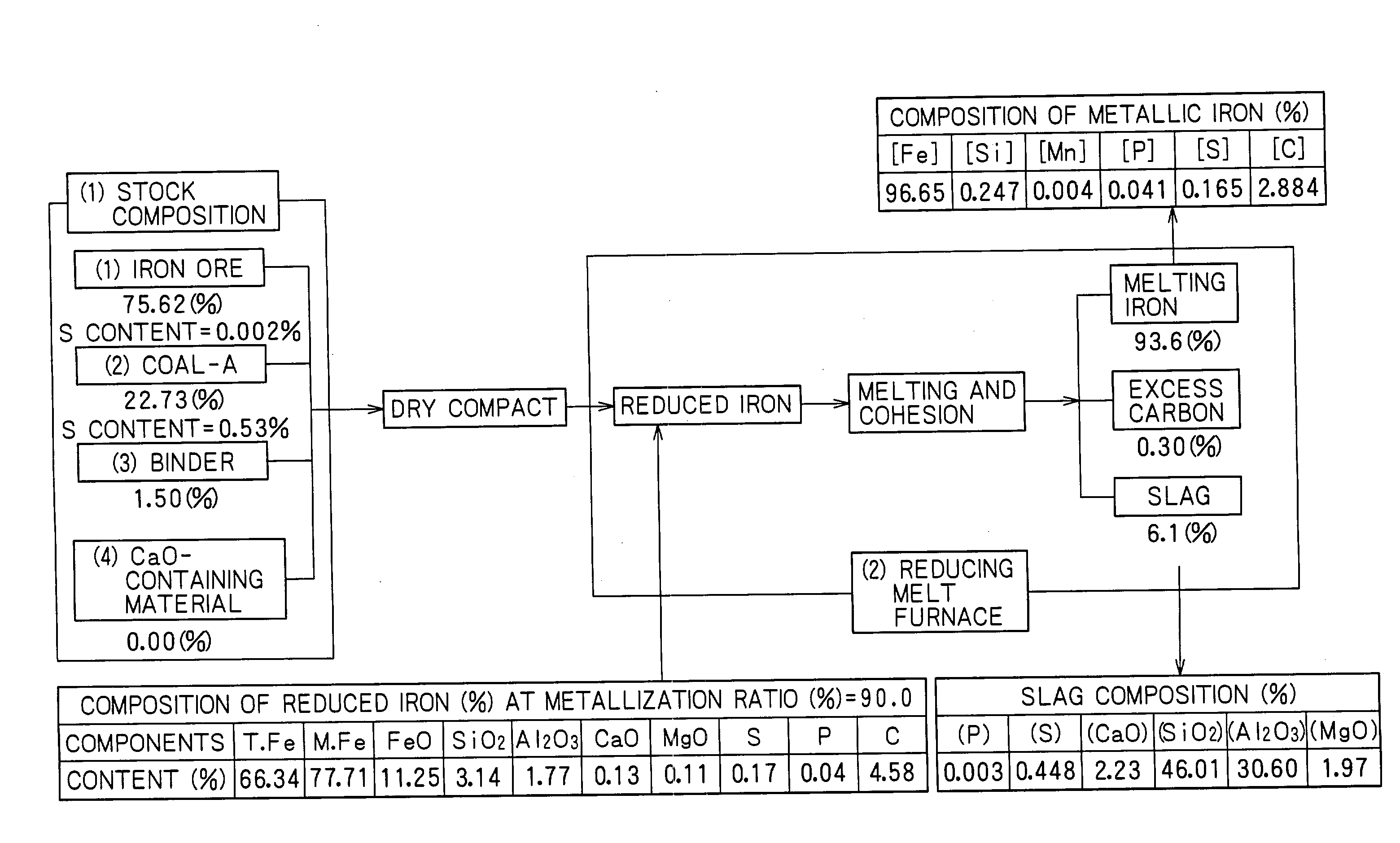
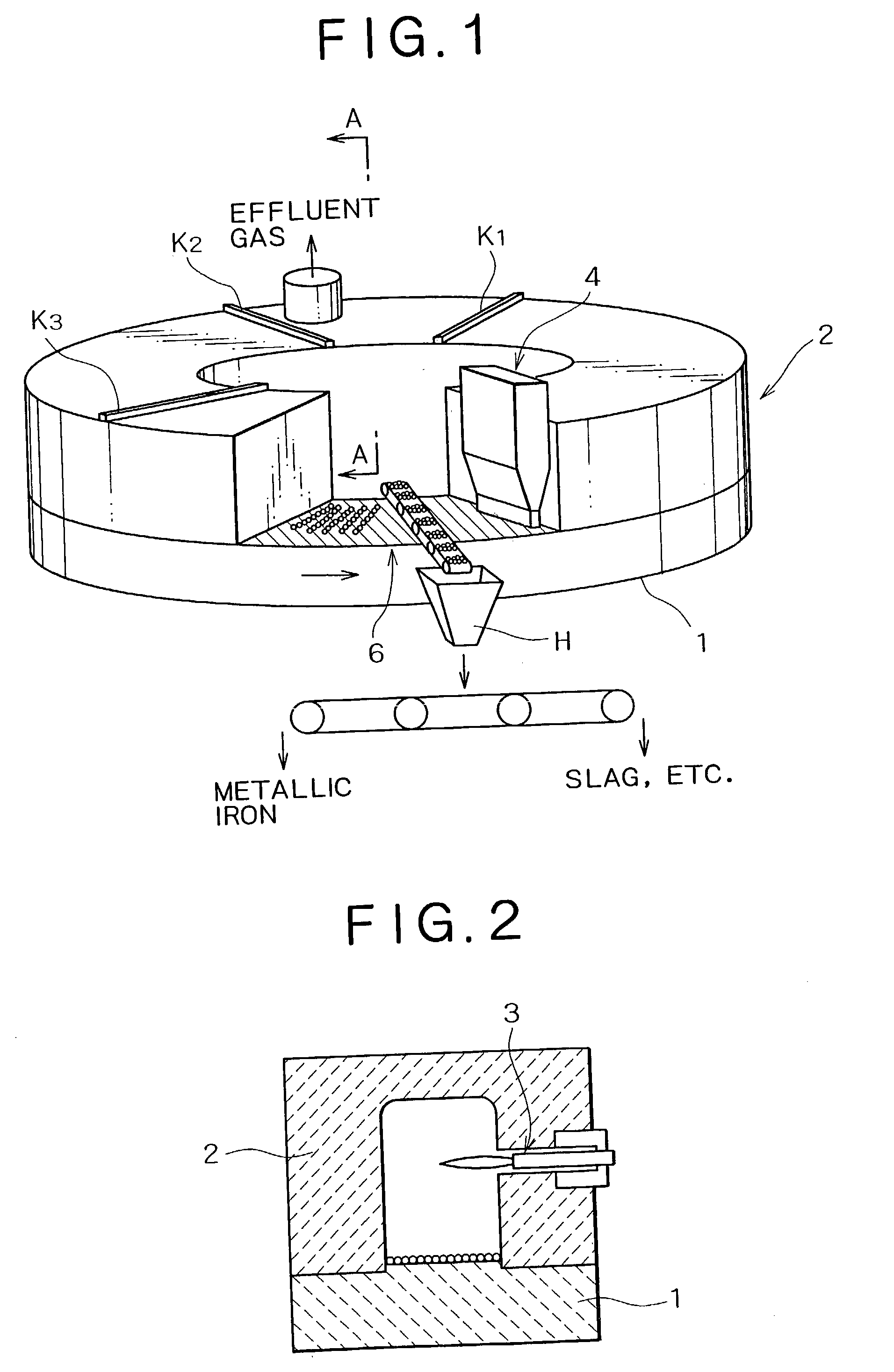
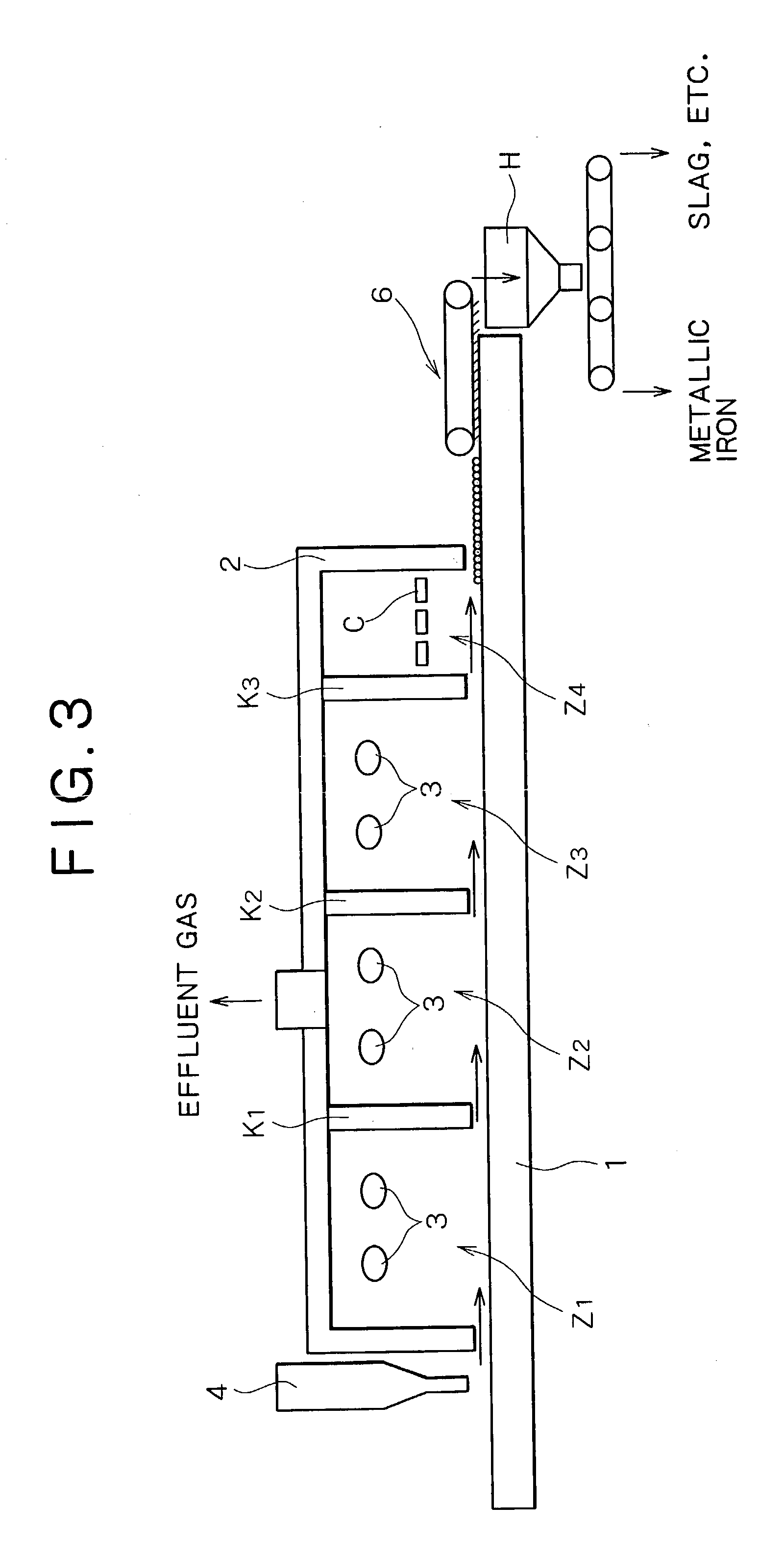
[0102] Material compacts having a diameter of approximately 19 mm were made by uniformly mixing hematite ore, i.e., an iron source, coal, and a small amount of a binder (bentonite). Metallic iron was made using these material compacts. The material compacts were fed inside a reducing-melt furnace of a rotary hearth type shown in FIGS. 1 to 3, and solid reduction was performed at an atmosphere temperature of approximately 1,350.degree. C. until a metallization ratio of approximately 90% was reached. Subsequently, the resulting material compacts were transferred to a carburization, melting, and cohesion zone at an atmosphere temperature of 1,440.degree. C. so as to perform carburization, melting, and cohesion, and to separate by-product slag to make slag-free metallic iron nuggets.
[0103] In this process, coal powder, i.e., an atmosphere adjustor, having a diameter of 2 mm or less was bedded on a hearth to a thickness of approximately 5 mm before the material compacts were fed to the f...
example 2
[0105] Material compacts having a diameter of approximately 19 mm were made by uniformly mixing magnetite ore, i.e., an iron source, coal, a small amount of a binder (bentonite), and 5% of CaCO.sub.3 as a slag basicity adjustor and forming the resulting mixture into compacts.
[0106] The material compacts were fed on a bed of coal powder (average diameter: approximately 3 mm) having a thickness of approximately 3 mm, the bed of coal powder being formed on a hearth. The coal powder was used as an atmosphere adjustor. The solid reduction was performed as in Example 1 while maintaining the atmosphere temperature at approximately 1,350.degree. C. until the metallization ratio reached nearly 100%. Subsequently, the resulting material compacts were transferred to a melting zone maintained at 1,425.degree. C. so as to perform carburization, melting, cohesion, and separation of by-product slag so as to make slag-free metallic iron. The material composition, the composition of the reduced iron...
example 3
[0112] An experiment was conducted under the same conditions as those in Example 1 and an actual furnace. In this experiment, the diameter of the material compacts (pellets) was varied within the range of 3 to 35 mm to examine the effect of the size of the material compacts on the average diameter and the average mass of the resulting metallic iron nuggets. The results are shown in FIG. 16.
[0113] As is apparent from this graph, metallic iron nuggets having a diameter in the range of 5 to 20 mm, i.e., the type of metallic iron nuggets exhibiting superior handling quality as the end-product metallic iron, could be effectively manufactured from material compacts (dry pellets) having a diameter of approximately 10 to 35 mm.
[0114] Industrial Applicability
[0115] The present invention having the above-described configuration provides metallic iron nuggets having a high Fe purity, an adequate C content, and a suitable size for handling ease. The metallic iron nuggets further has low S, Si, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com