Device and method for treating conditions of a joint
a technology of joint and joint pain, applied in the direction of osteosynthesis devices, prostheses, surgical staples, etc., can solve the problems of increased likelihood of such pain-causing ailments, increased pain, bone spurs and limited movement, and increased time lost by productive individuals due to pain, especially in the joints
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
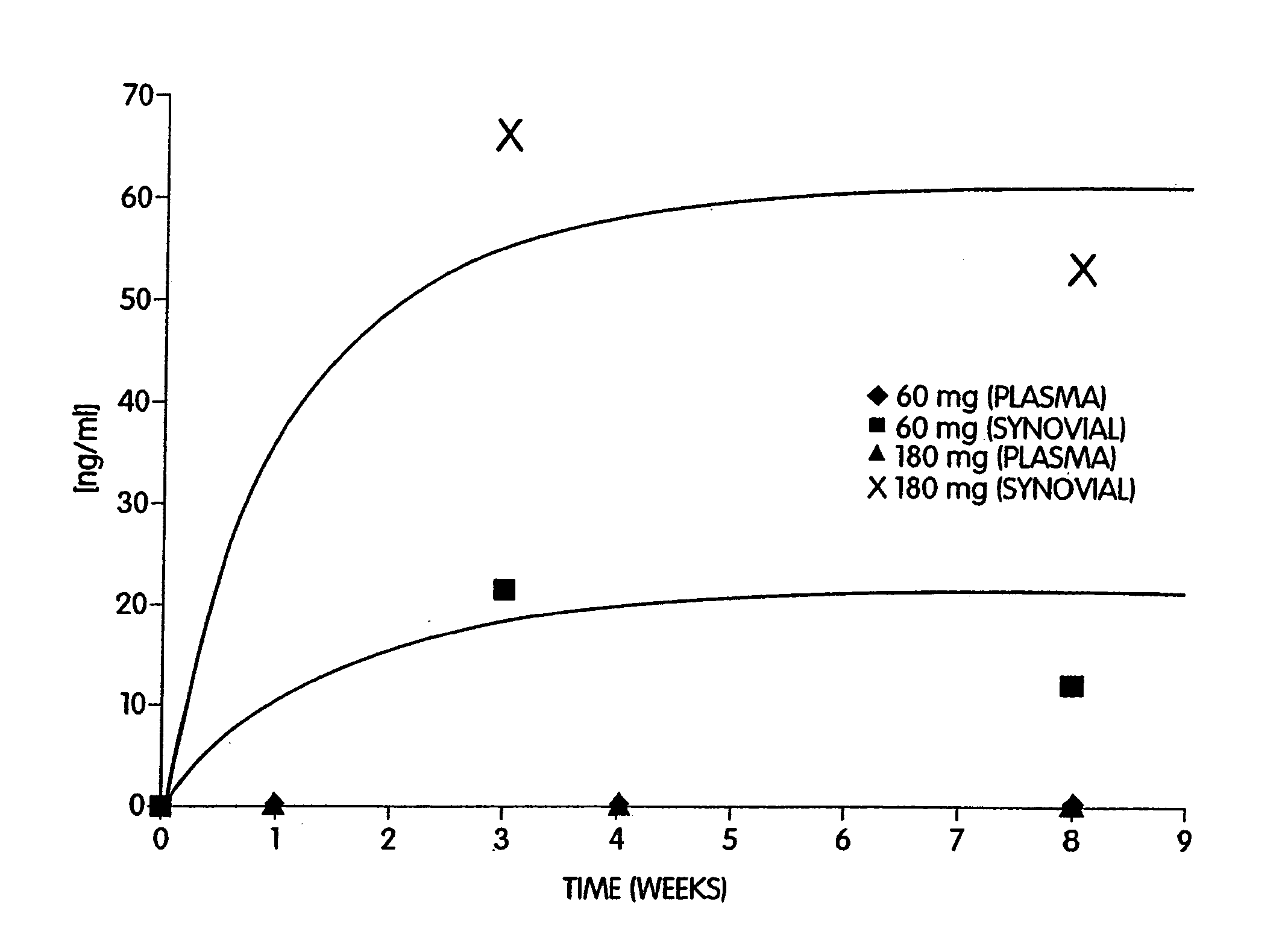
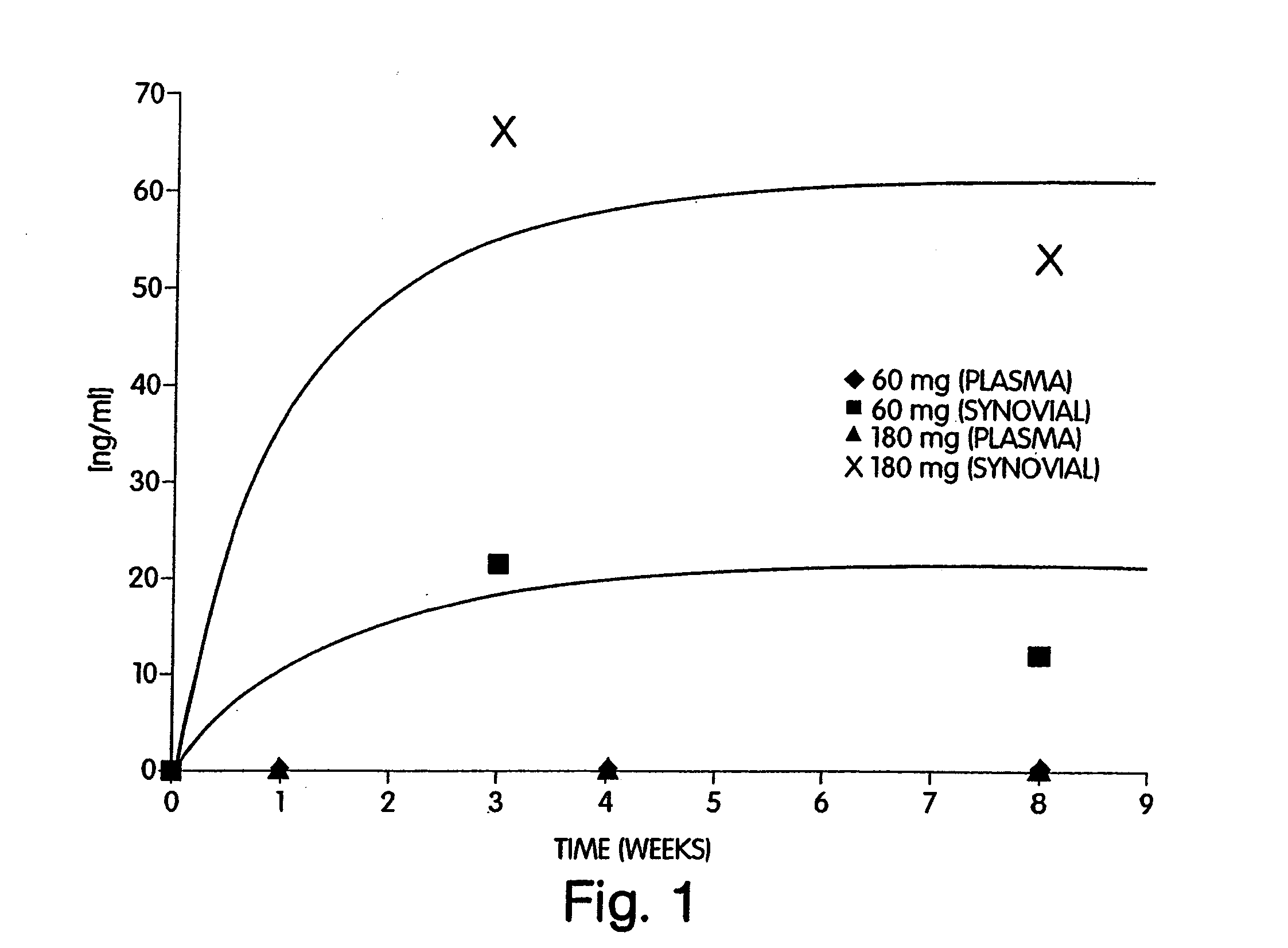
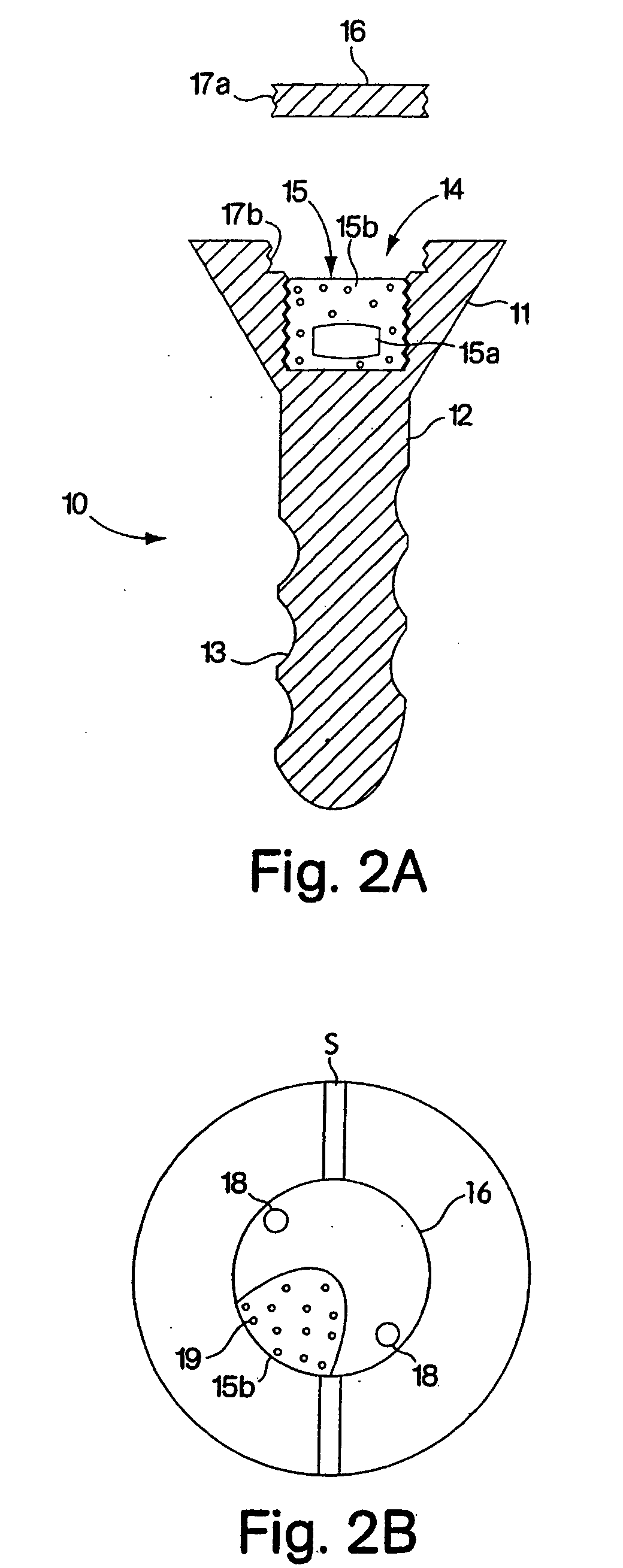
In one embodiment, the present invention provides a method for administering a therapeutically effective compound to the synovial fluid of a joint. The method comprises the step of implanting a sustained release device within the joint such that the therapeutically effective compound is released within the synovial capsule. The synovial fluid concentration of the compound remains greater than plasma concentration for the lifetime of the sustained release device. In one aspect of the invention, the synovial fluid concentration of the compound remains at least one order of magnitude greater than the plasma concentration. In a preferred aspect of the invention, the synovial fluid concentration of the compound remains several orders of magnitude greater than the plasma concentration.
Additional applications of the systems described herein will become readily apparent to those skilled in the art from the following detailed description, wherein various embodiments of the invention are d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com