Decontamination of prion-contaminated surfaces with phenols
a technology of phenols and prion, applied in the field of biological decontamination, can solve the problems of ineffective breakdown, tissue damage, cell death, etc., and achieve the effect of deactivating prions quickly and effectively and facilitating instruments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Study of the Effect of Phenol Concentration on the Effectiveness of the Composition
To test the contribution of various formulation effects on the priocidal activity of various compositions experiments are performed using IFDO log reduction as the response. The ingredients of compositions I-VII are listed in Table 1. Composition I is a commercial formulation, LpH™.
The IFDOs are artificially cultured in a modified Mycoplasma broth and quantified by serial dilutions and plating on a similar agar. The efficacy of the compositions I-VII is studied by suspension testing at room temperature at a 1% dilution of the composition in water. Following a suitable contact time, e.g., 10 minutes, aliquots are sampled and quantified by serial dilution and plating into modified Mycoplasma agar. Following incubation at 37° C. for 48 hours, the plates are evaluated by counting visible colonies and log reductions are determined. Results with the compositions are compared with an existing phenolic pr...
example 2
Effect of Approximately Equimolar Concentrations of Phenols
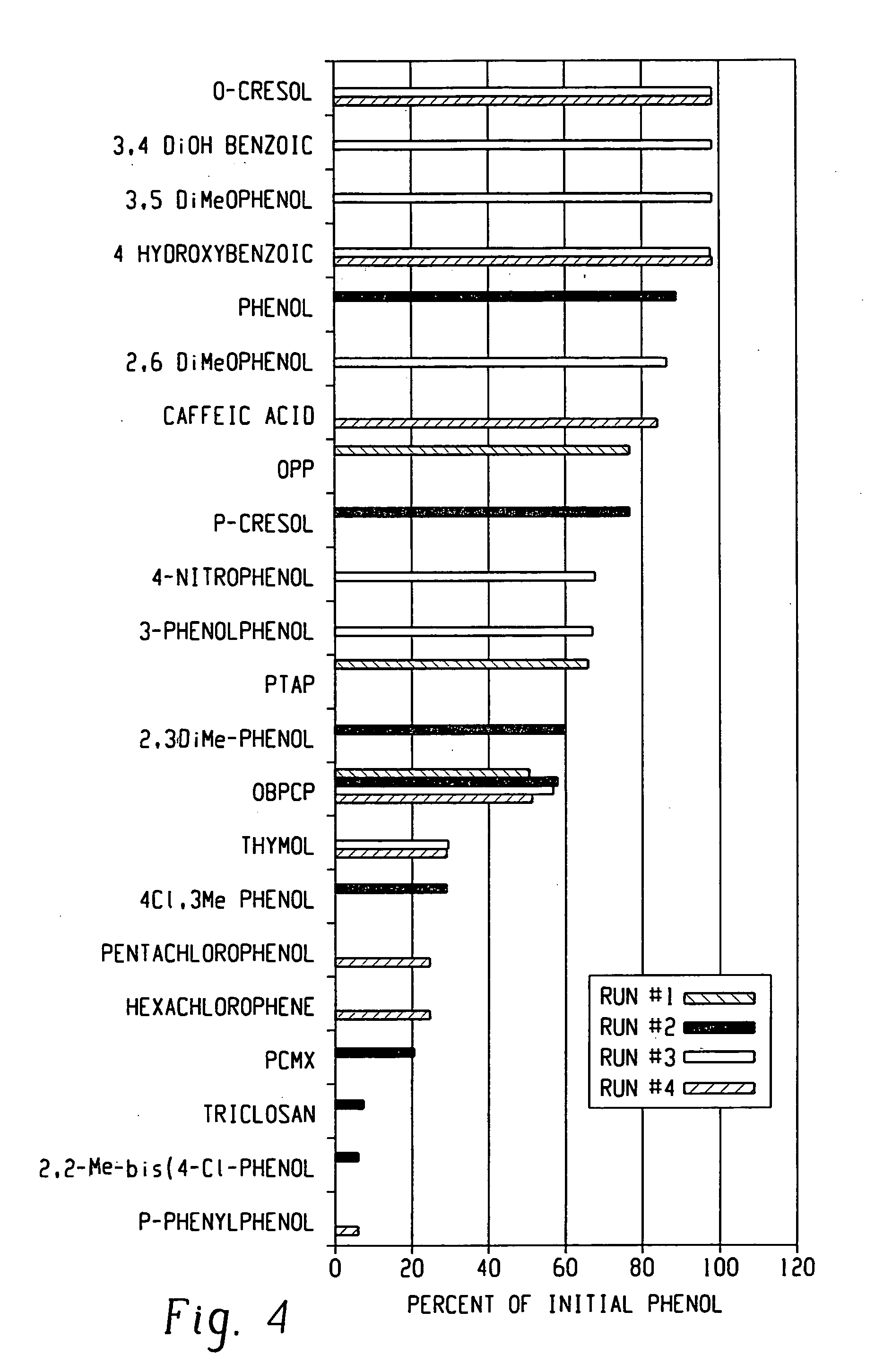
Various phenols at approximately equimolar concentrations (where possible when solubility permitted) are studied by the method of EXAMPLE 1. TABLE 2 shows the ingredients by weight for formulations IX-XX and the results obtained.
TABLE 2MolMolecularPhenol / Ingredientwt100 gIXXXIXIIXIIIXIV2,3-Dimethylphenol122.170.09011.00o-Benzyl-p-218.690.08618.86Chlorophenolo-Phenylphenol142.580.08414.29p-Chloro-m-Cresol156.610.08712.45p-Chloro-m-Xylenol150.20.09915.502,4,5-197.460.09017.80TrichlorophenolHexylene Glycol4.003.956.294.214.004.23iso-Propyl alcohol8.007.907.628.148.408.08Sodium22.4618.8620.6019.9219.8019.60LaurylsulfateAlpha olefin6.706.326.106.037.006.45sulfonateGlycolic Acid19.0018.6817.1418.3021.0018.00Triethanolamine2.501.430.951.341.401.02Soft Water26.3424.0027.0129.6122.9024.82Log Reduction4.14.74.84.34.44.9MolMolecularPhenol / Ingredientwt100 gXVXVIXVIIXVIIIXIXXX2,2-Methylenebis1220.0516.17(4-chlorophenol)Hexachlorophen...
example 3
Correlation of Results with Partition Coefficients (Pc)
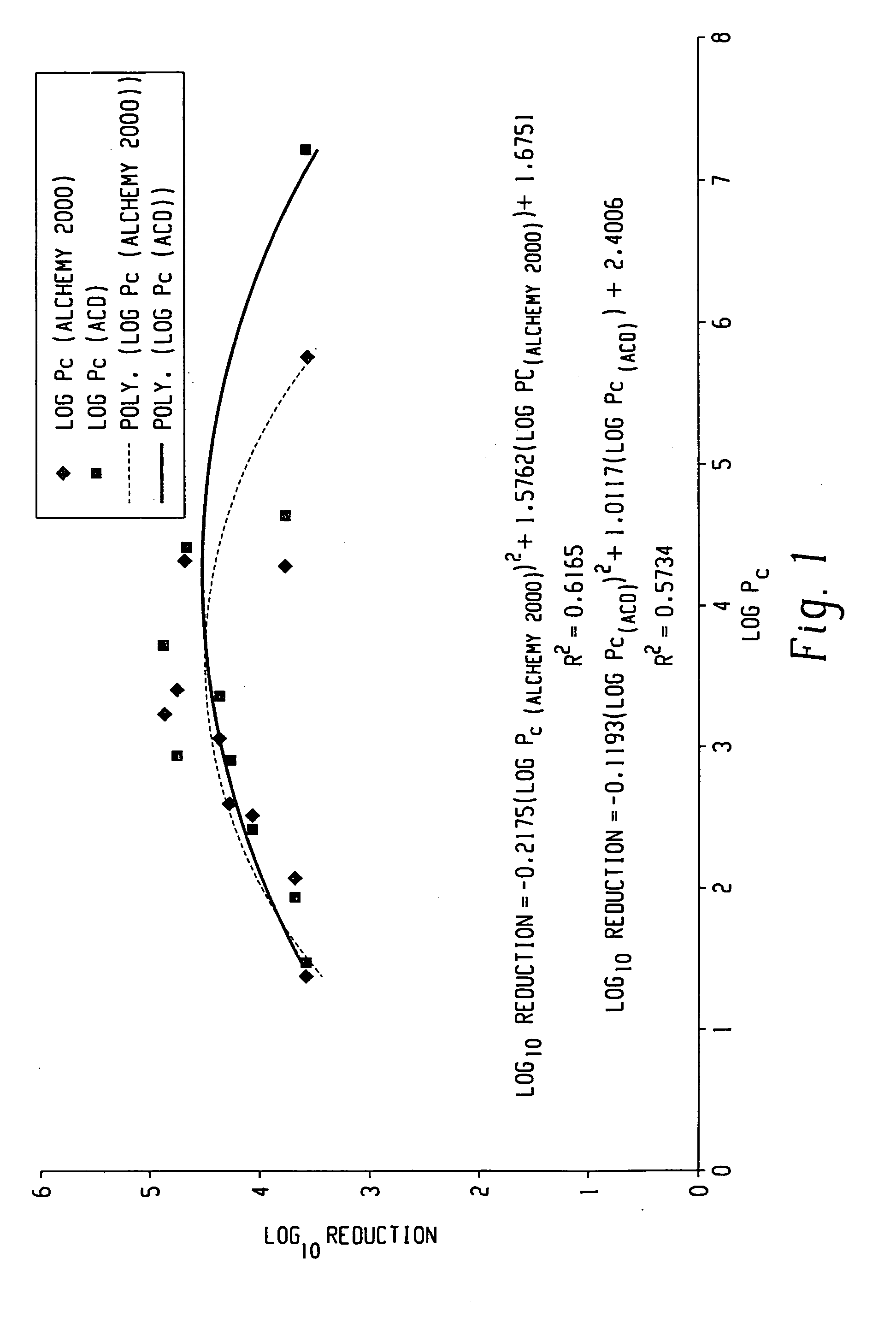
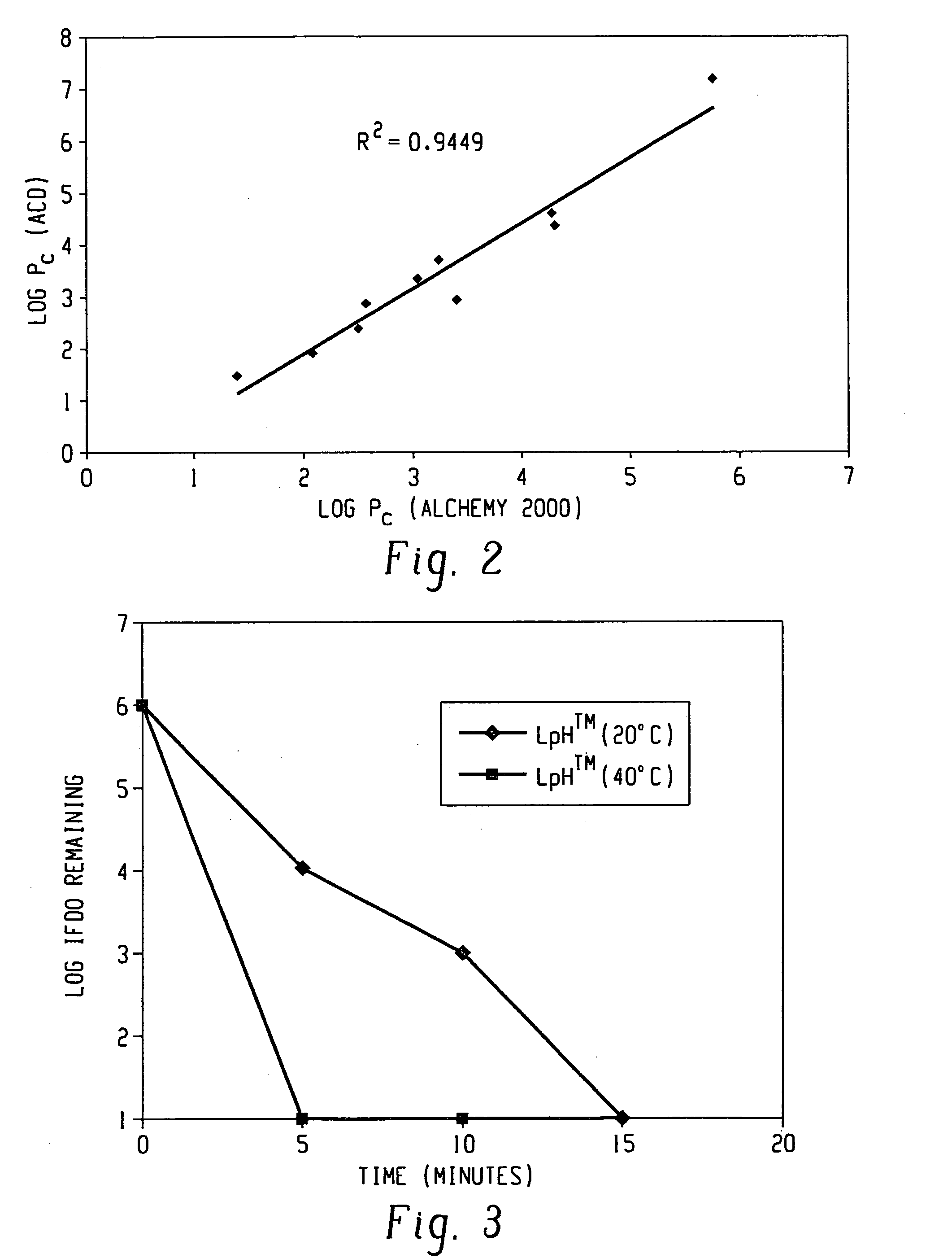
Pc is defined as the calculated octanol-water partition coefficient. The log Pc values are calculated using two methods. The first method uses Alchemy 2000 Molecular Modeling Software (Tripos) along with a data set developed by STERIS Corporation. The second method uses Advanced Chemistry Development (ACD) Software Solaris v4.67 (© 1994-2002 ACD). The calculated log Pc values for each phenol are shown in TABLE 3: These values are compared with Log reduction colonies obtained in Example 2.
TABLE 3Log Pc(AlchemyLog10 ReductionPhenol2000)Log Pc (ACD)of ColoniesPhenol1.391.483.6p-Cresol2.081.943.72,3-Dimethylphenol2.502.404.1p-Chloro-m-cresol2.582.894.3p-Chloro-m-xylenol3.053.354.42,4,5-Trichlorophenol3.233.714.9Thymol3.273.283.2o-Phenylphenol3.402.944.82,2-Methylenebis(4-4.274.623.8chlorophenol)o-Benzyl-p-chlorophenol4.324.414.7Triclosan4.515.822.7Hexachlorophene5.757.203.6
FIG. 1 shows the Log IFDO reduction vs Log Pc (Alchemy 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com