Method for quantitating triglycerides in lipoproteins
a technology of lipids and triglycerides, applied in the field of lipid quantitation methods, to achieve the effect of convenient quantitation of triglycerides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0058]
Measurement of TG in LDLReagent 1 (pH 6.25)Buffer [Piperazine-1,4-bis(2-50mMethanesulfonic acid)(PIPES)]TOOS (Dojindo Laboratories)0.3g / LATP 2Na salt (Wako Pure Chemical2.5g / LIndustries, Ltd.)Ascorbic acid oxidase3kU / L(Asahi Kasei Corporation)GK (Toyobo Co., Ltd.)1kU / LGPO (Asahi Kasei Corporation)8kU / LPeroxidase (Toyobo Co., Ltd.)20kU / LPEG modified LPL1.5kU / L(Toyobo Co., Ltd.)LPL III (Amano)60kU / LNonion NS-230 (NOF Coporation)0.1%Magnesium sulfate heptahydrate0.5g / L(Wako Pure ChemicalIndustries, Ltd.)Reagent 2 (pH 6.25)Buffer (PIPES)50mMEmulgen 7090.6%Triton DF-160.3%4-Aminoantipyrine0.5g / L(Wako Pure ChemicalIndustries, Ltd.)
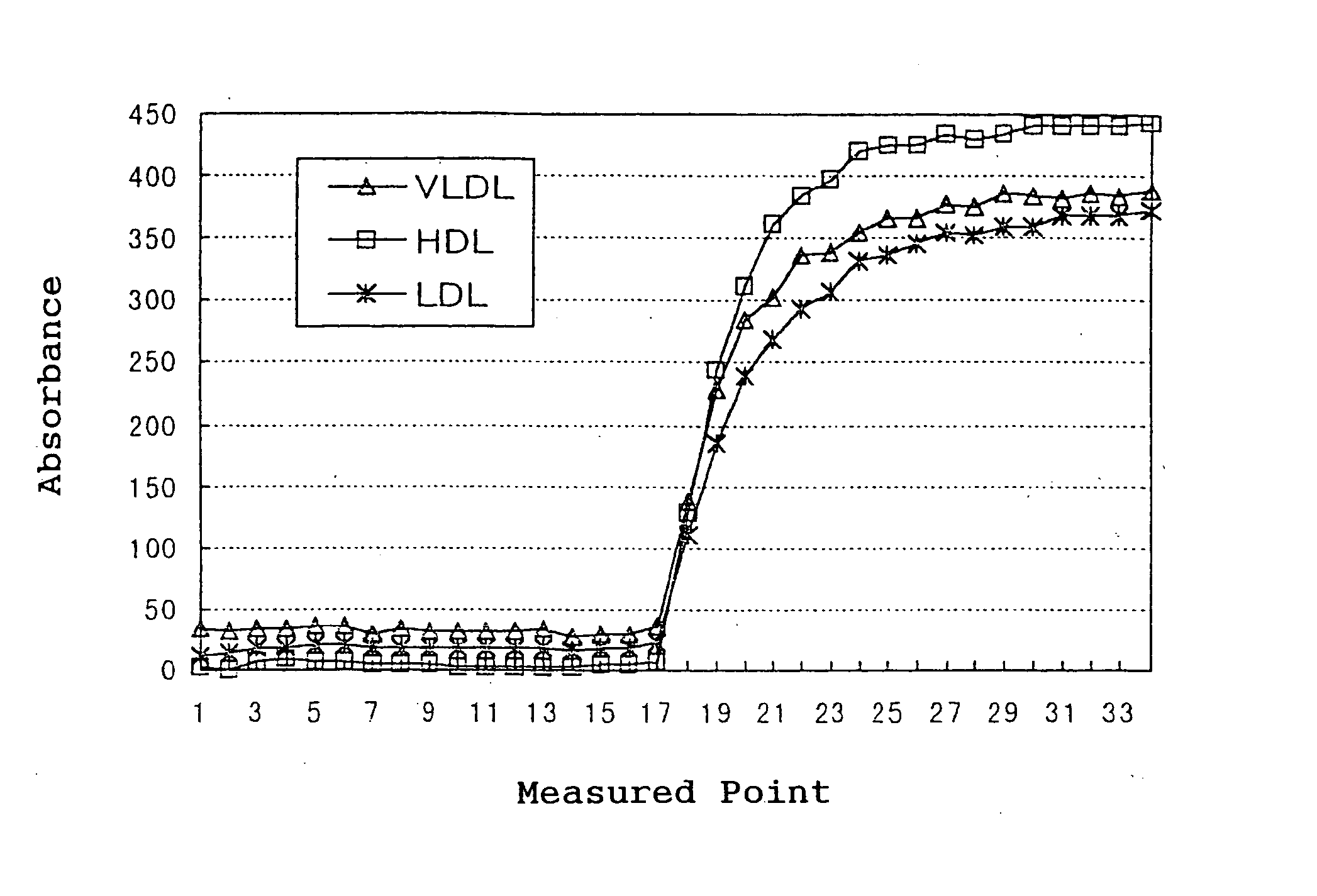
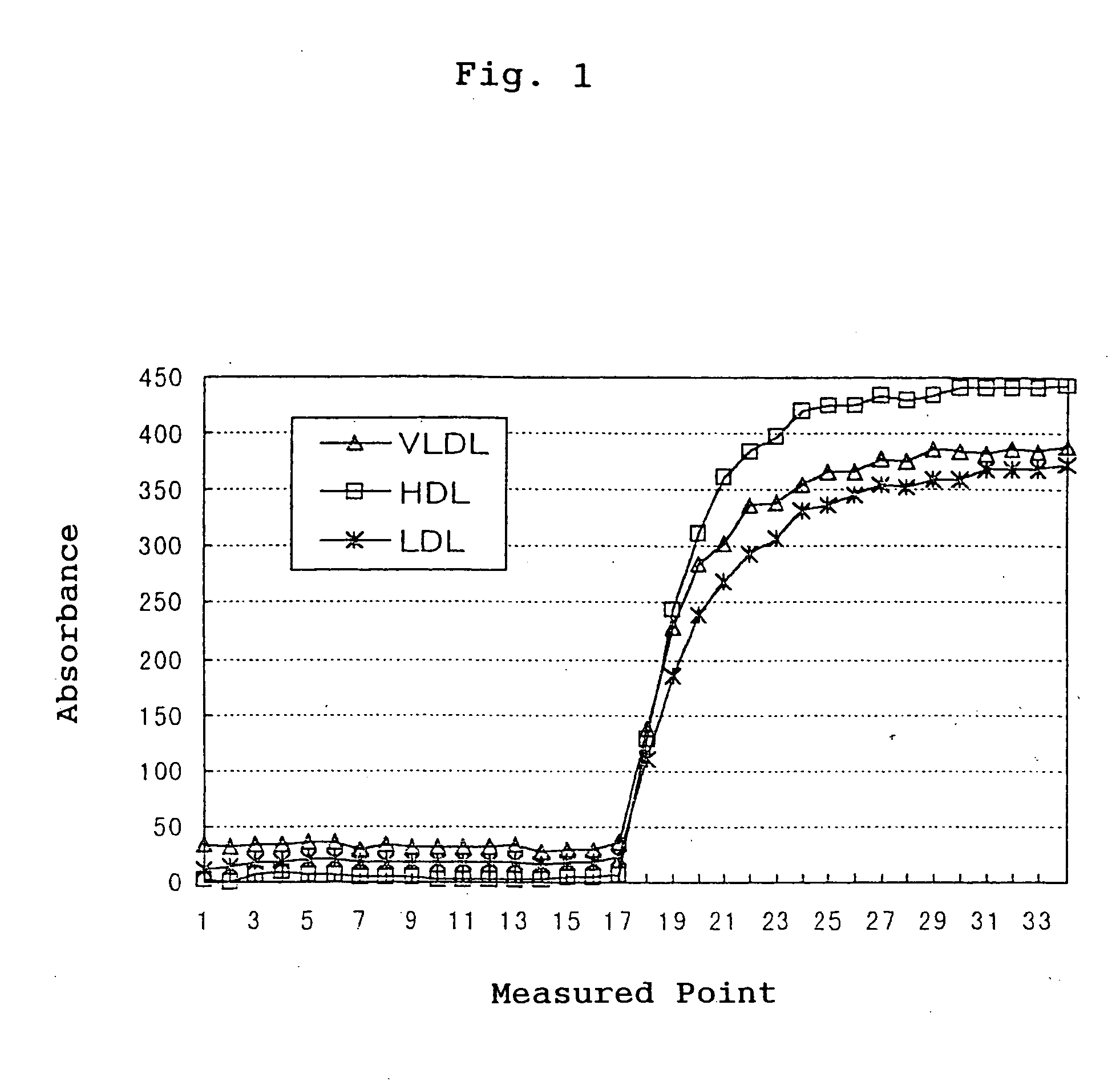
[0059] The specificity was confirmed by tracing the time course under the condition of the following parameters using a Hitachi Auto-Analyzer 7170. As a sample, HDL, LDL and VLDL fractions fractionated from human serum by ultracentrifugation were used. FIG. 1 shows the time course measured with a reagent for measuring total TGs (Kyowa Medex Co., Ltd.) for ...
example 2
[0069]
Measurement of TG in HDLReagent 1 (pH 6.25)Buffer (PIPES)50mMTOOS (Dojindo Laboratories)0.3g / LATP 2Na salt (Wako Pure2.5g / LChemical Industries, Ltd.)Ascorbic acid oxidase3kU / L(Asahi Kasei Corporation)GK (Toyobo Co., Ltd.)1kU / LGPO (Asahi Kasei Corporation)8kU / LCatalase (Sigma)300kU / LSodium dextransulfate0.2g / LMagnesium sulfate heptahydrate0.5g / L(Wako Pure ChemicalIndustries, Ltd.)Reagent 2 (pH 6.25)Buffer (PIPES)50mMEmulgen B-6620g / L(Kao Corporation)Calcium chloride dihydrate0.1g / L4-Aminoantipyrine0.5g / L(Wako Pure ChemicalIndustries, Ltd.)Sodium azide0.5g / LLPL (Asahi Kasei Corporation)1000kU / LPeroxidase (Toyobo Co., Ltd.)20kU / L
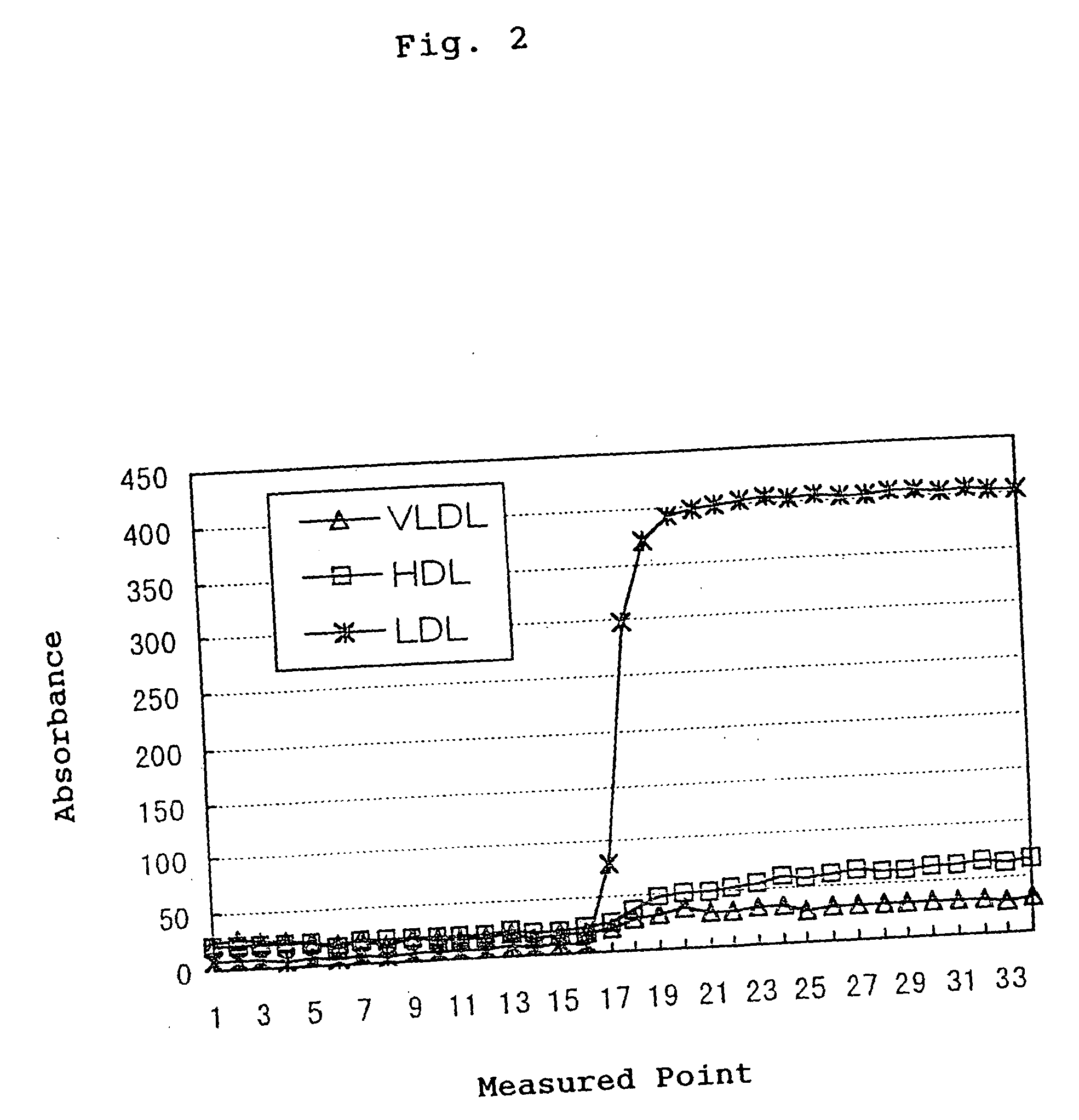
[0070] The specificity was confirmed by tracing the time course under the condition of the following parameters using a Hitachi Auto-Analyzer 7170. As a sample, the same HDL, LDL and VLDL fractions fractionated from human serum by ultracentrifugation as Example 1 were used. As shown in FIG. 3, in treatment with the reagent 1 in the first reaction, free gl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| TG | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com