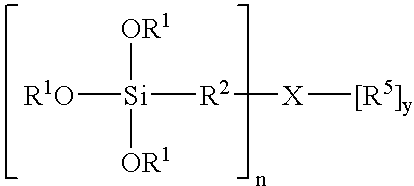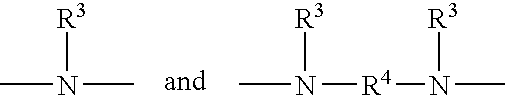Silane compositions and methods for bonding rubber to metals
a technology of compositions and compositions, applied in the field of silane coatings for treating metals, can solve the problems of difficult application, cure, control, and less viscosity of silanes, and achieve the effects of efficient and economical adhesion of polymeric compositions, minimal waste, and easy application and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
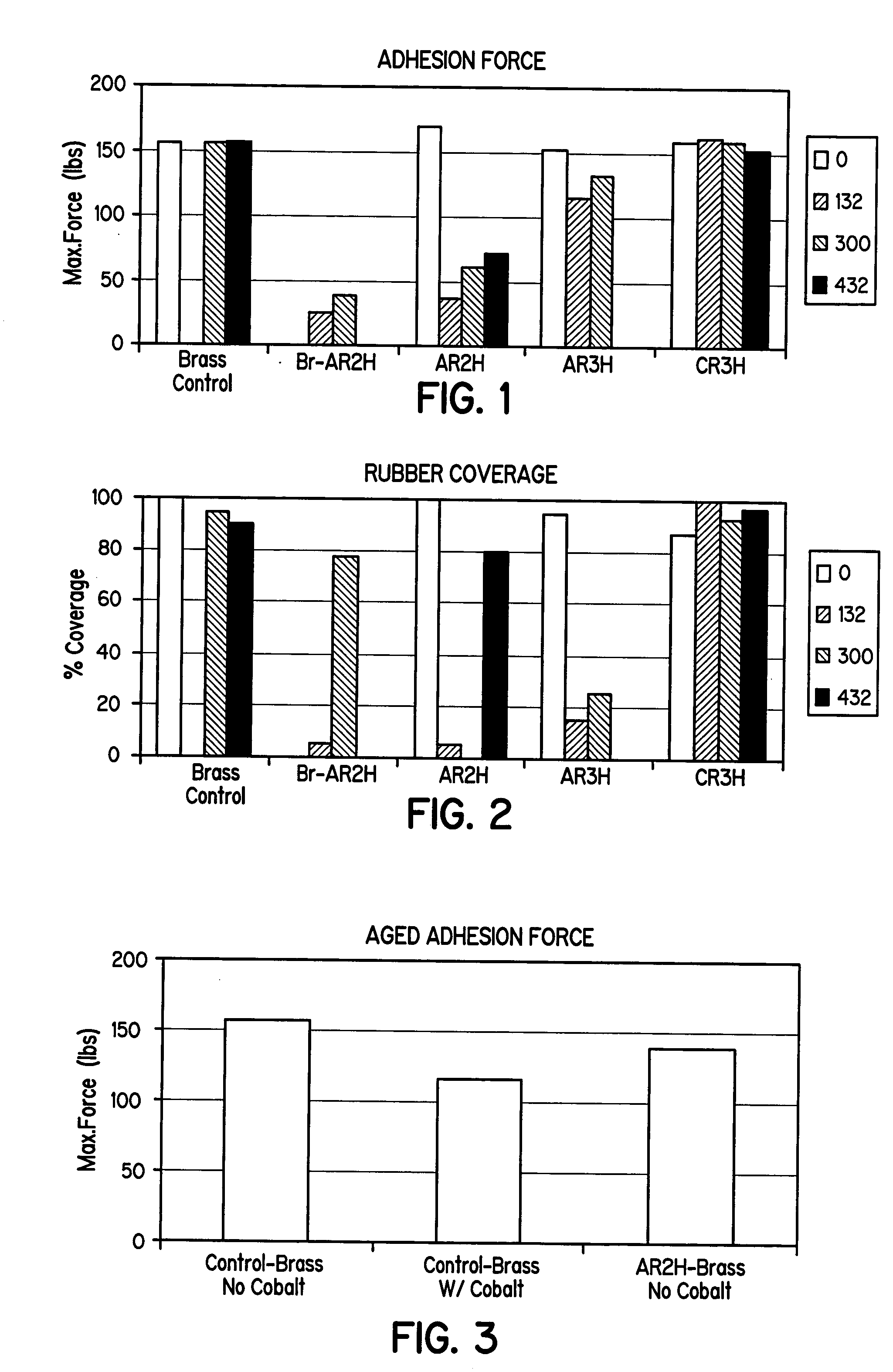
FIGS. 1, 2, and 3 illustrate the bonding results (maximum force and rubber coverage) between natural rubber and silane-coated brass metal panels subjected to the ASTM 429-B test after the following aging periods: 0 hours, 132 hours, 300 hours, and 432 hours, at 70° C. The “control” samples are blank, un-coated brass-metal panels.
FIGS. 1 and 2 illustrate beneficial concentration ratios between the aminosilane and the sulfur-containing silane as it relates to bond strength. As shown, ratios of about 1:1 to about 1:3 provide advantages in bond strength. Also, the silane solution C generally performed better than solution A in the ASTM 429-B tests. Solution C, i.e., the combination of substantially hydrolyzed Y9400 and A1589, provided as good or better bond strength and adhesion force as the control, but without the inclusion of cobalt additives in the rubber composition. Solution A, also provided good adhesion relative to the control sample, but without the inclusion of cobalt additiv...
example 2
Table I provides maximum force (lbs) and rubber coverage (%) results of natural rubber-to-metal bonds for zinc-plated brass metal panels subjected to the ASTM 429-B test after the aging period. The solutions were prepared and the tests were preformed in accordance with that described above.
TABLE ISilaneRubberSolutionAgingCobaltMax. ForceCoverage(5%)Period (hrs)(Yes / No)(lbs)(%)AR2H24Y14.50AR2H24N138.495CR3H24Y115.080CR3H24N17085Control24Y175100Control24N169.690AR2H48Y13.15AR2H48N133.780CR3H48Y41.810CR3H48N130.775Control48Y184.995Control48N140.285AR2H132Y405AR2H132N170.390AR3H132Y114.715CR3H132Y165.7100CR3H132N145.9100Control132N126.3100AR2H300Y62.50AR2H300N153.995AR3H300Y132.425CR3H300Y167.895CR3H300N143.885Control300Y156.460Control300N133.895AR2H432Y72.0380AR2H432N125.385CR3H432Y138.2100CR3H432N148.3100Control432Y147.9100Control432N107.790
The discrepancies in the data reported above are due, in part, to the nature of the method of measuring adhesion. For example, many of the sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com