Arginyl-glutamine dipeptide for treatment of pathological vascular proliferation
a technology of arginylglutamine and dipeptide, which is applied in the direction of peptide/protein ingredients, drug compositions, and metabolic disorders, can solve the problems of impaired vision, many challenges for people with visual impairment, and inability to fully enjoy the visual aspects of their surroundings, so as to prolong the life of diabetics and achieve the effect of high survival rate of very premature infants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Use of Arginyl-glutamine to Prevent Retinopathy of Prematurity
[0100] Neonatal mice were exposed to a period of hyperoxia to induce retinal angiogenesis that mimics retinopathy of prematurity. An Arginyl-glutamine dipeptide was administered intraperitoneally twice daily during the period when neovascularization occurs in this model, days 12 through 17.
[0101] On day 17 the animals are sacrificed and degree of angiogenesis was quantified by counting pre-retinal neovascularization on stained sections from treated and untreated animals.
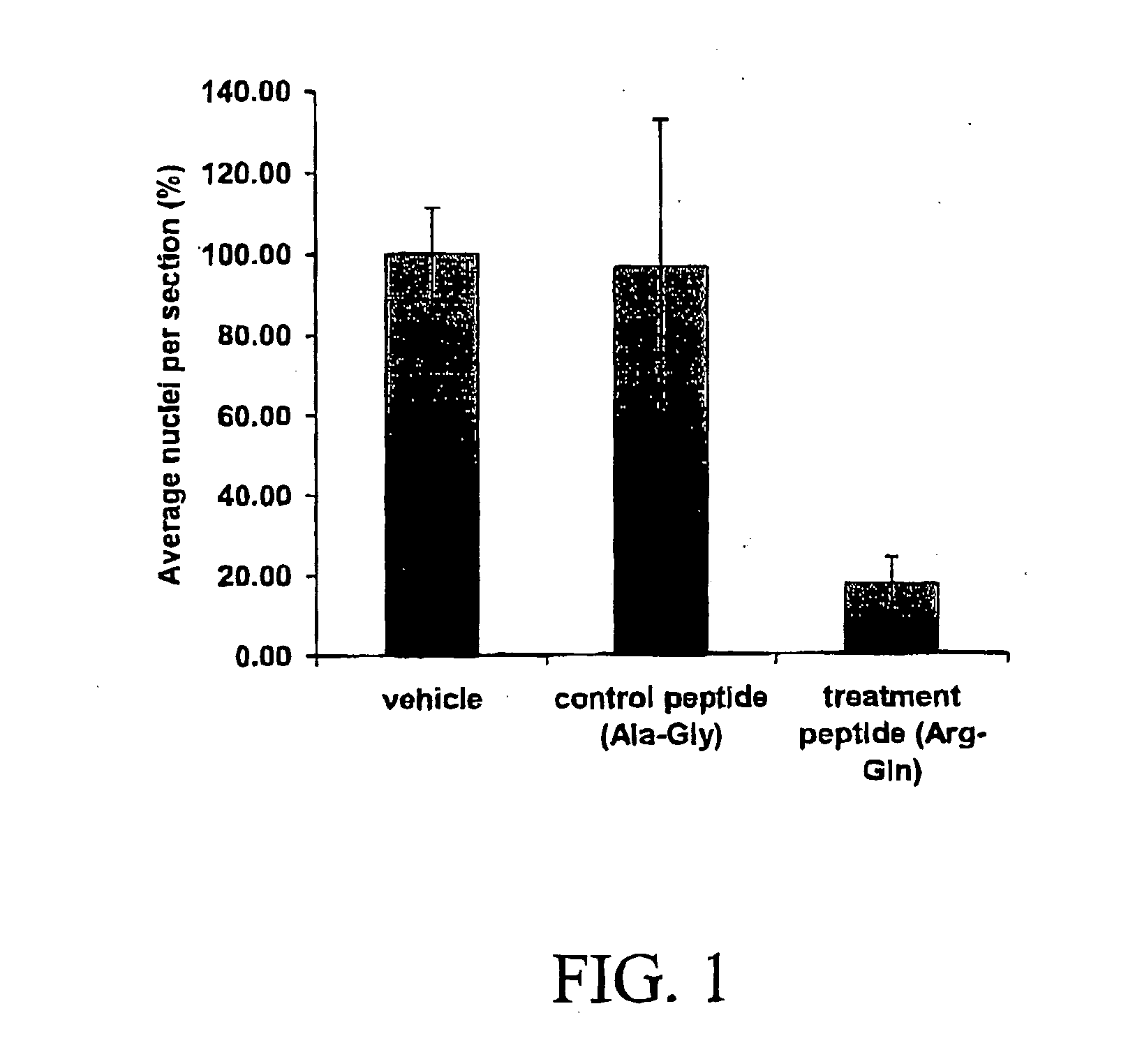
[0102] The data summarized in FIG. 1 represent combined data from two separate experiments. The results demonstrated a statistically significant effect of the dipeptide (argininyl-glutamine) compared to vehicle and dipeptide (alaninyl-glycine). Treatment of the pups with the dipeptide (argininyl-glutamine) resulted in a 80% reduction in preretinal neovascularization.
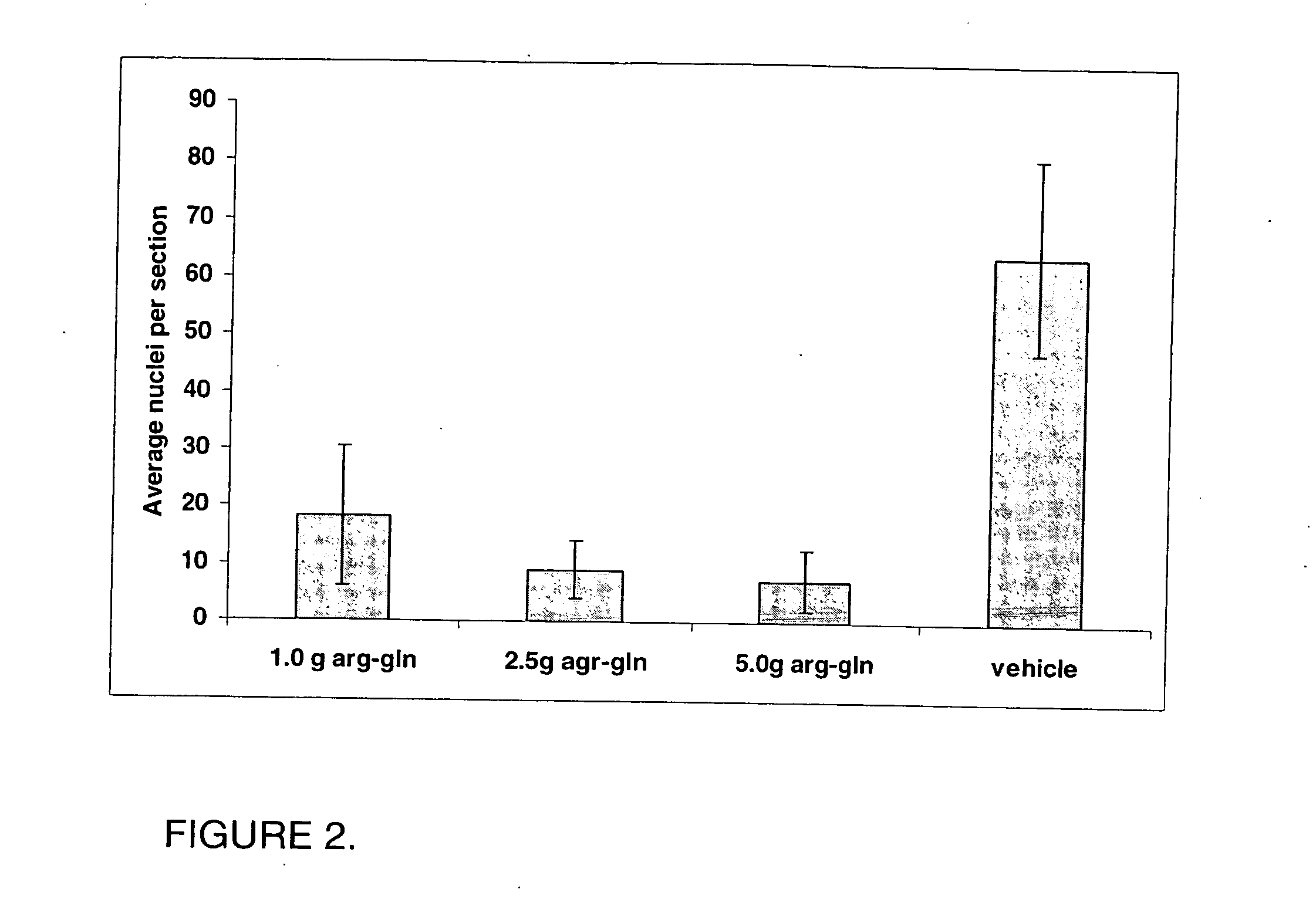
[0103] This experiment has been repeated with different doses of the dipeptide compared...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| Molecular Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com