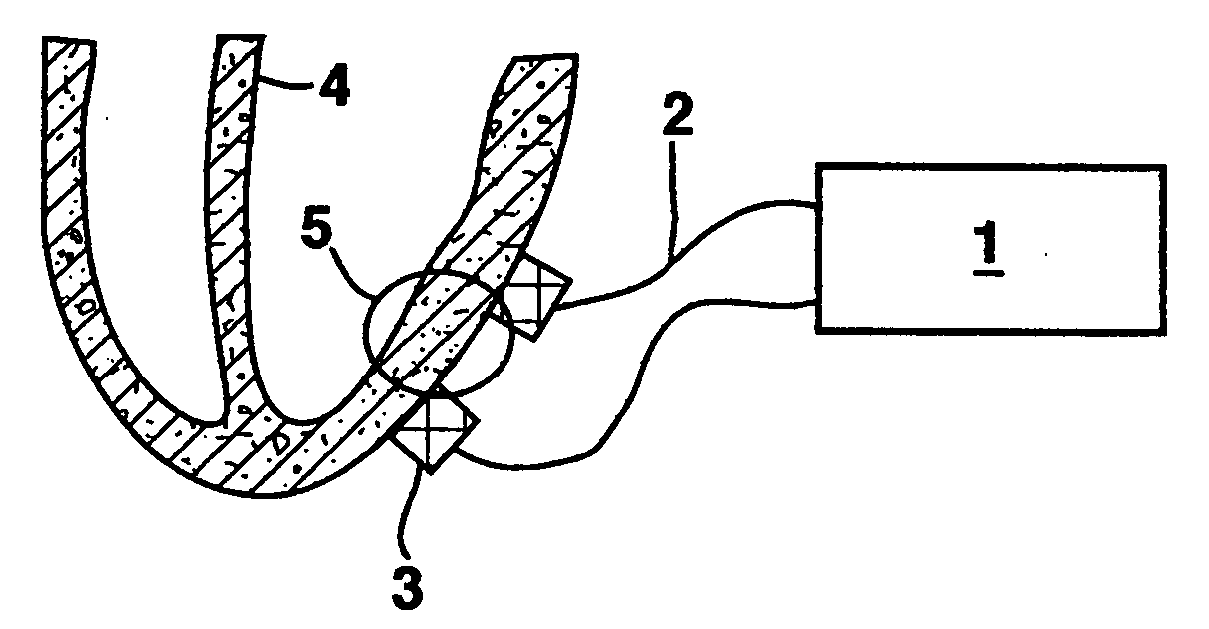
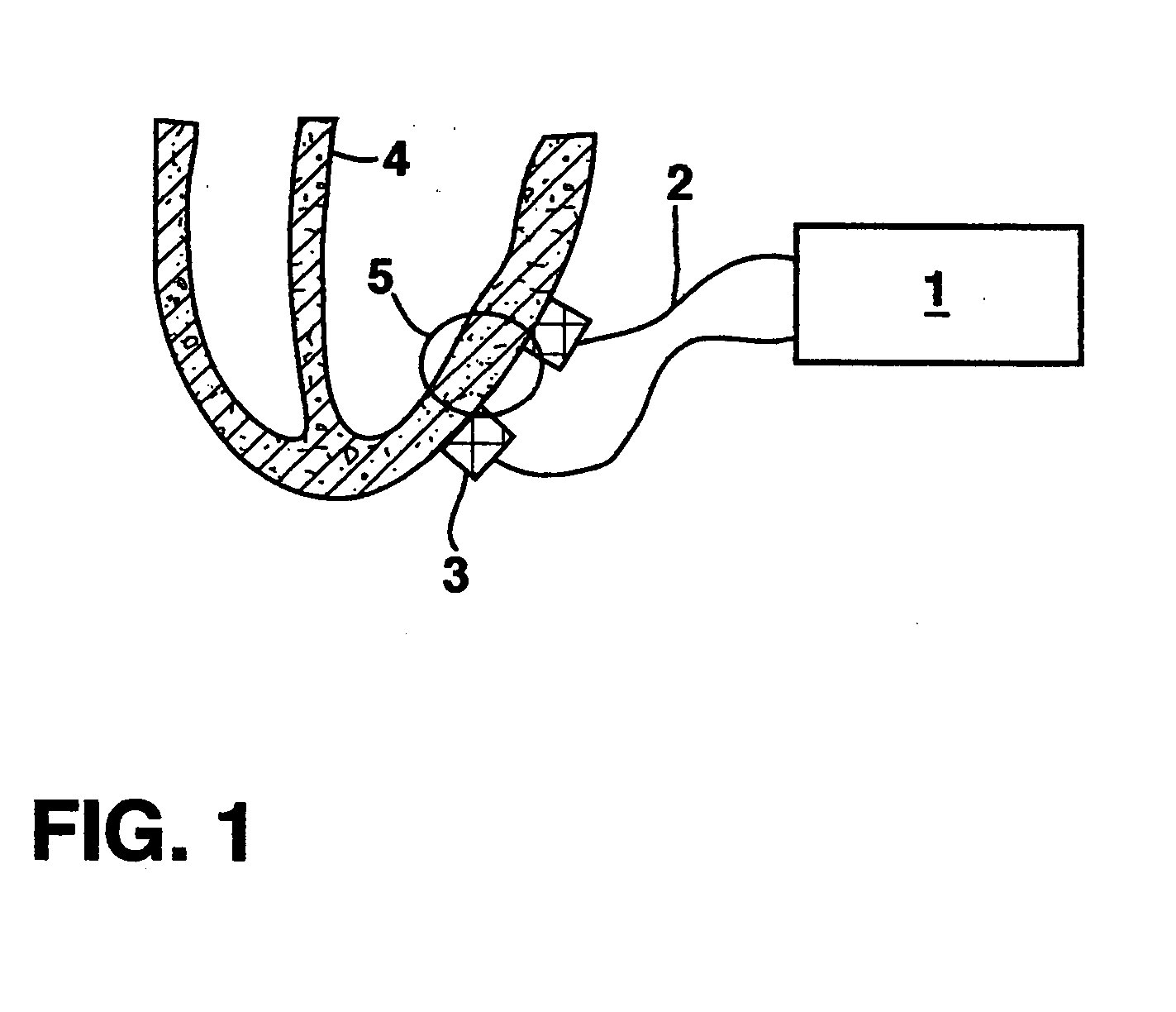
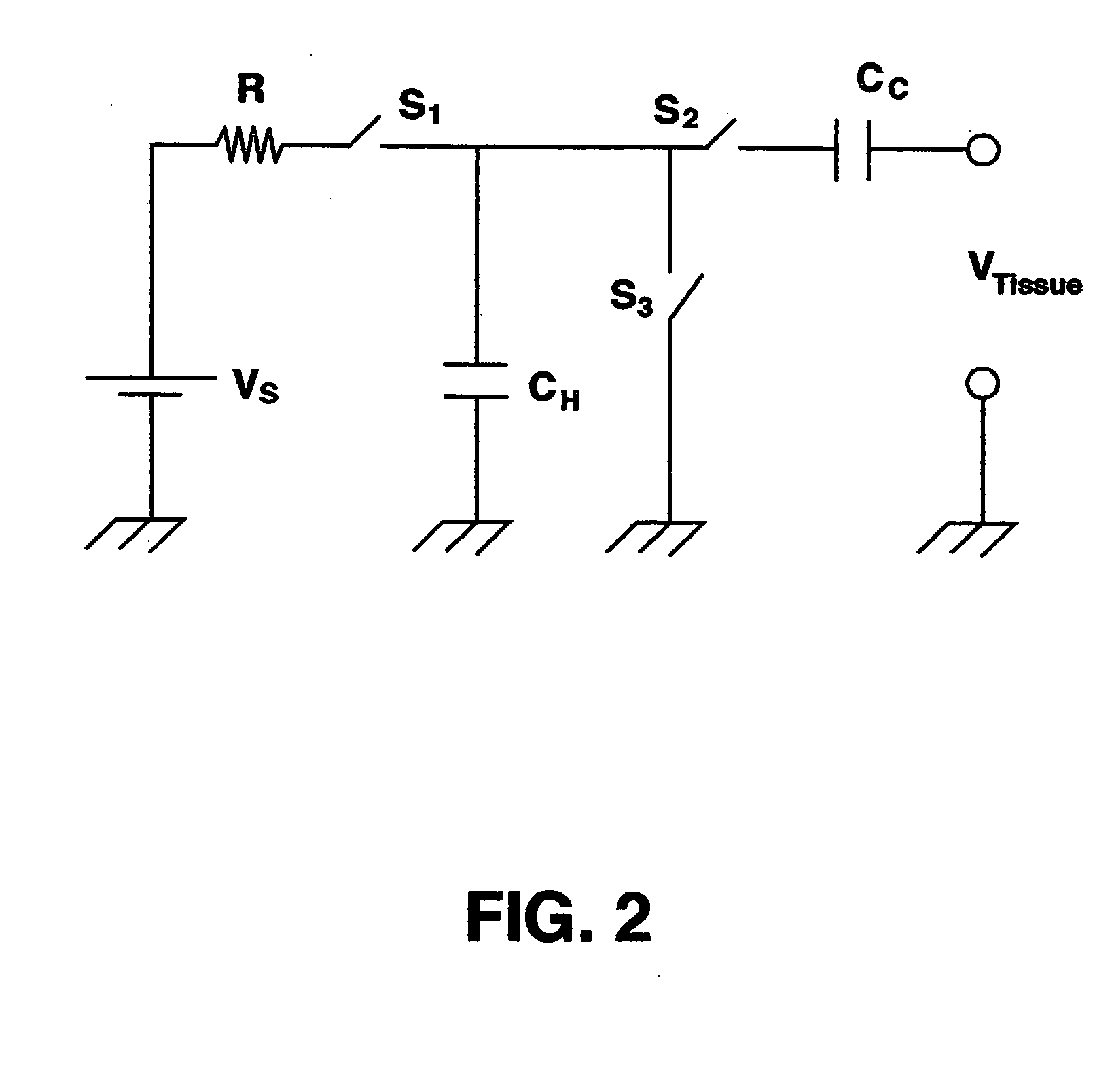
Stimulation for delivery of molecular therapy
a technology stimulatory device, which is applied in the field of stimulatory device for the controlled production of angiogenic growth factor, can solve the problems of ischemic heart area, occurrence of myocardial infarction, and starvation of oxygen and nutrients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0091] Sterile 6 well culture plates (Corning) were seeded with cells on six well culture inserts using SmGM growth media; C2C12 cells (mouse myoblasts) were seeded at 7.5×103 / cm2; Human Coronary Smooth Muscle Cells (HCASMC) were seeded at 2.5×103 / cm2. After two days' growth (confluent), the wells were washed twice with electrical stimulation medium (DMEM with 1% bovine serum albumin). 2.0 ml of serum free medium was added but without any fetal bovine serum in the medium. Wells contained approximately 4 ml of total growth medium, approximately 2.0 ml inside well insert and 2.0 ml outside well insert. The cells were electrically stimulated using a circular graphite electrode for 8 hours per stimulation condition. Cells were stimulated at one volt in the stimulation chamber for 1 msec stimulation pulse width with a 4 msec discharge pulse width. The escape period was adjusted to achieve the desired frequency. Samples were harvested after 22 hours post-stimulation. Cell culture supernat...
example 2
In Vivo Subthreshold Stimulation in Canine Model of Regional Ischemic Cardiomyopathy
[0093] Dogs were initially anesthetized with intramuscular morphine sulfate (4 mg / kg) / A bolus injection of pentothal (20 mg / kg) was given followed by continuous inhaling of isoflurane (0.5%-2% in oxygen) after endotracheal intubation. A left lateral thoracotomy was performed, and the pericardium opened. A micromanometer pressure transducer (MPC-500, Millar Instruments, Houston, Tex.) was inserted into the left ventricle through an apical incision. Pairs of 5 MHz ultrasonic crystals also was implanted in the area supplied by the distal left anterior descending (LAD) and left circumflex (LCX) arteries just distal to the first diagonal branch of measurement of coronary flow. Two heart wire electrodes were inserted in the LAD perfusion area. A catheter was inserted into the left atrium for injection of colored 15 um microspheres to measure regional myocardial blood flow. Ameriod constrictors were placed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com