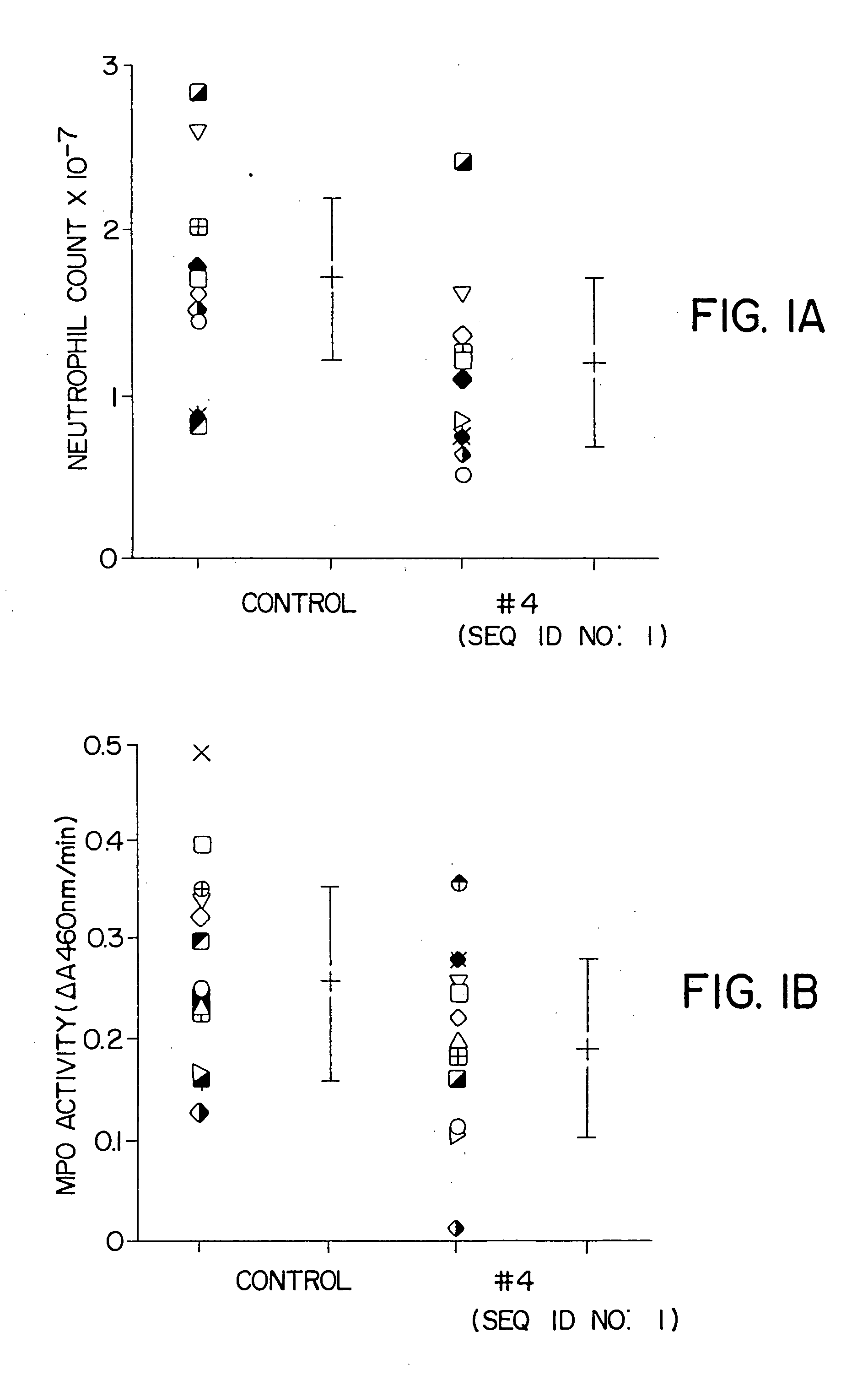
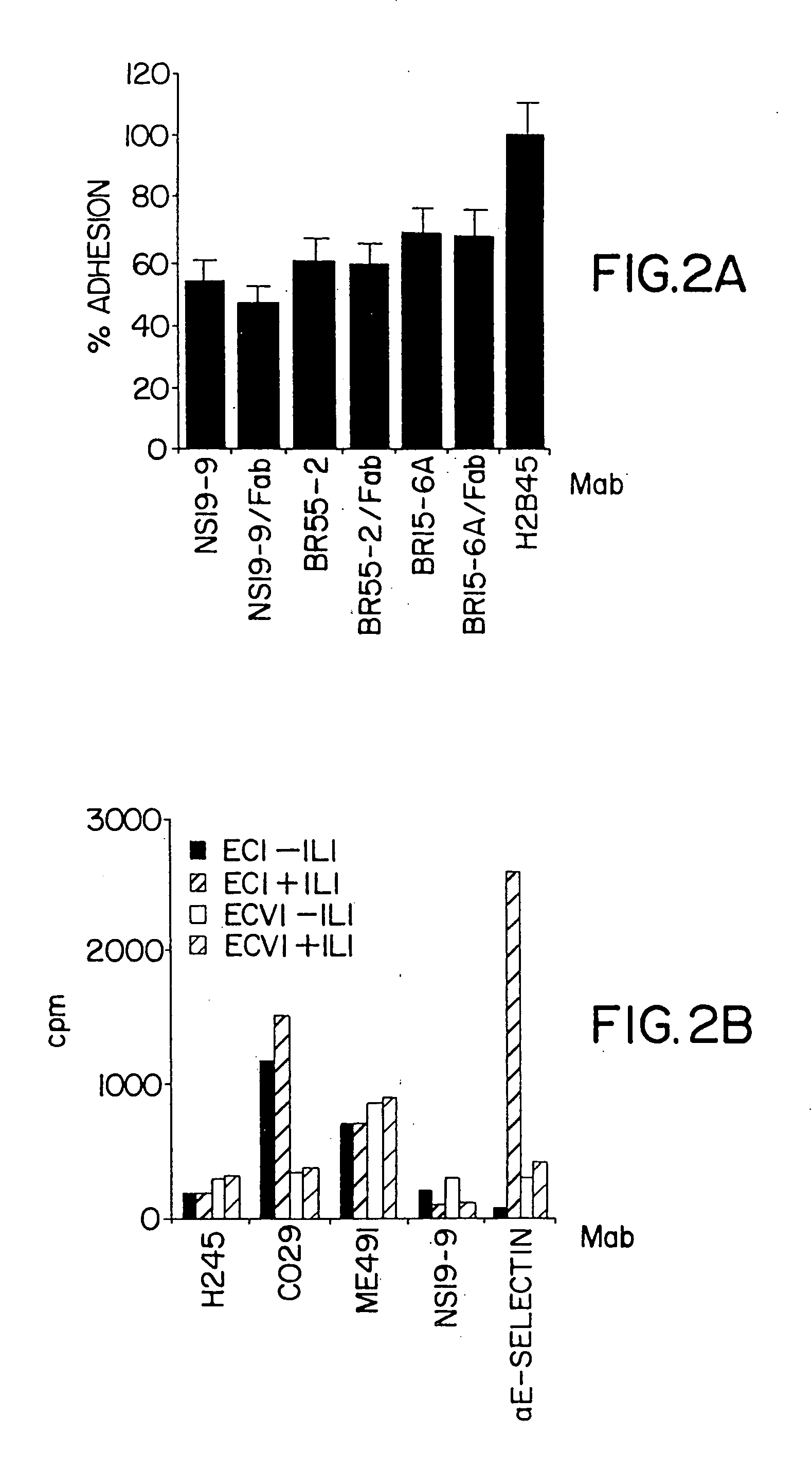
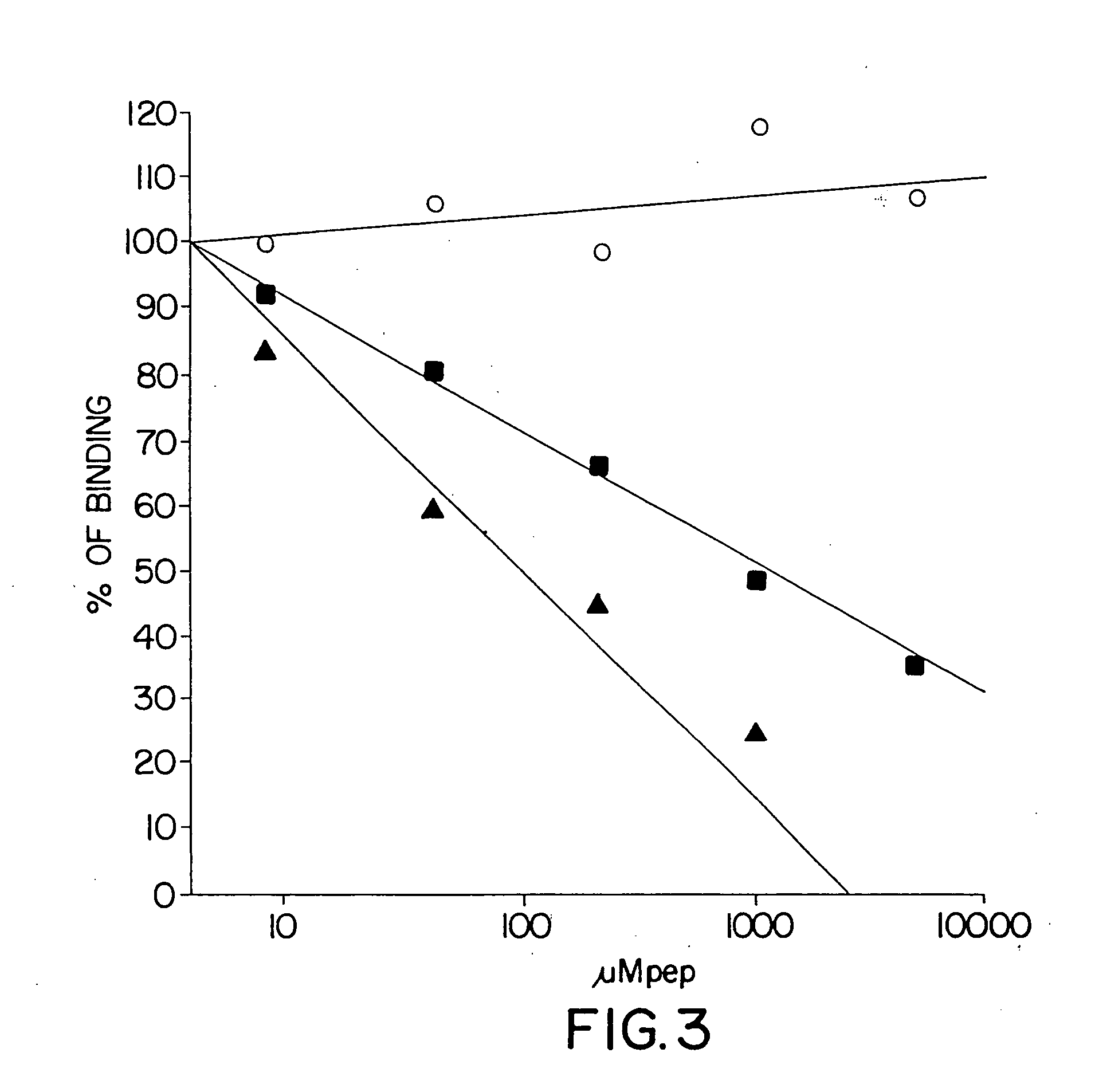
Compositions and methods for treatment of cancer
a technology of adhesion protein and peptide, which is applied in the field of animal cell adhesion protein peptidomimetics, can solve the problems of long time-consuming and laborious treatment of cancer, affecting the development of anti-adhesion therapy for the treatment of disease processes for which there is no effective treatment, and affecting the study of these processes. it can reduce the adhesion of tumor cells and reduce the metastasis of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Screening of a Random Peptide Library
[0091] To develop novel molecules to inhibit the adhesion of human adenocarcinoma cells to EC and, ultimately, to inhibit metastasis in vivo, peptides were derived from a 12-mer random peptide library. A diverse library of random dodecapeptides, displayed as flagellin-thioredoxin fusion proteins (FLITRX) on the surfaces of E. Coli bacterial cells, was obtained from Invitrogen (Carlsbad, Calif.) [LaVallie et al., 1993, Bio / Technology, 11:187-193]. This library enables efficient isolation of bacteria displaying peptides with affinity to immobilized antibodies or to other binding proteins. The use of this library offered advantages over phage display in which the level of expression of phage coat protein genes is low and the selected peptides are usually unconstrained molecules with many degrees of conformational freedom. Moreover, no phage infection or isolation steps are necessary using, the FLITRX fusion protein system. Such highly diverse pepti...
example 2
Protein Expression, Isolation and Sequencing
[0093] In the final selection cycle described above, the clones were tested for protein expression using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting [Towbin et al., 1979, Proc. Natl. Acad. Sci. USA 76:4350-4354] as follows. Specifically, each bacterial pellet was collected and dissolved directly in SDS-PAGE sample buffer containing SDS and P-mercaptoethanol. Proteins were separated using 12% polyacrylamide gel and transferred into nitrocellulose filters according to standard procedures. Expression of the FLITRX fusion protein-containing peptide sequences mimicking carbohydrate structures expressed by the bacterial clones was detected using one of the following monoclonal antibodies (10 pg / ml): [0094] (1) MAb BR15-6A, which is specific for the carbohydrate ligand LeY [Rodeck et al., 1987, Hybridoma 6:389-401], [0095] (2) MAb NS19-9, which is specific for the carbohydrate ligand SA-Lea [...
example 3
Competition Enzyme-Linked Immunosorbent Assay
[0102] The peptide competition / inhibition assays referred to throughout the following examples are performed as follows: Test peptides at concentrations ranging from 10 nM to 1 mM were preincubated with 100 μl of the MAb NS19-9 (5 μg / ml) diluted in 10% μ-globulin free horse serum / PBS at room temperature. Fifty μl of preincubated inhibition complex antibody-peptide is added to each well of 96-well plate and incubated at 30-37° C. for 1 hour. After 1 hour of incubation, MAb / peptide complex mixtures were transferred to wells precoated with a constant amount of neoglycoprotein containing coupled multivalent carbohydrate determinant (SA-Lea-polyacrylamide matrix (SA-LeX-PAA or LeY-PAA) (5 μg / well) and allowed to bind for 1 hour followed by blocking with 10% γ-globulin-free horse serum for 2 hours at room temperature. Wells were washed with 100 μl PBS four times. Goat anti-mouse immunoglobulin G conjugated to horse radish peroxidase (Boehringe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Adhesion strength | aaaaa | aaaaa |
| Stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com