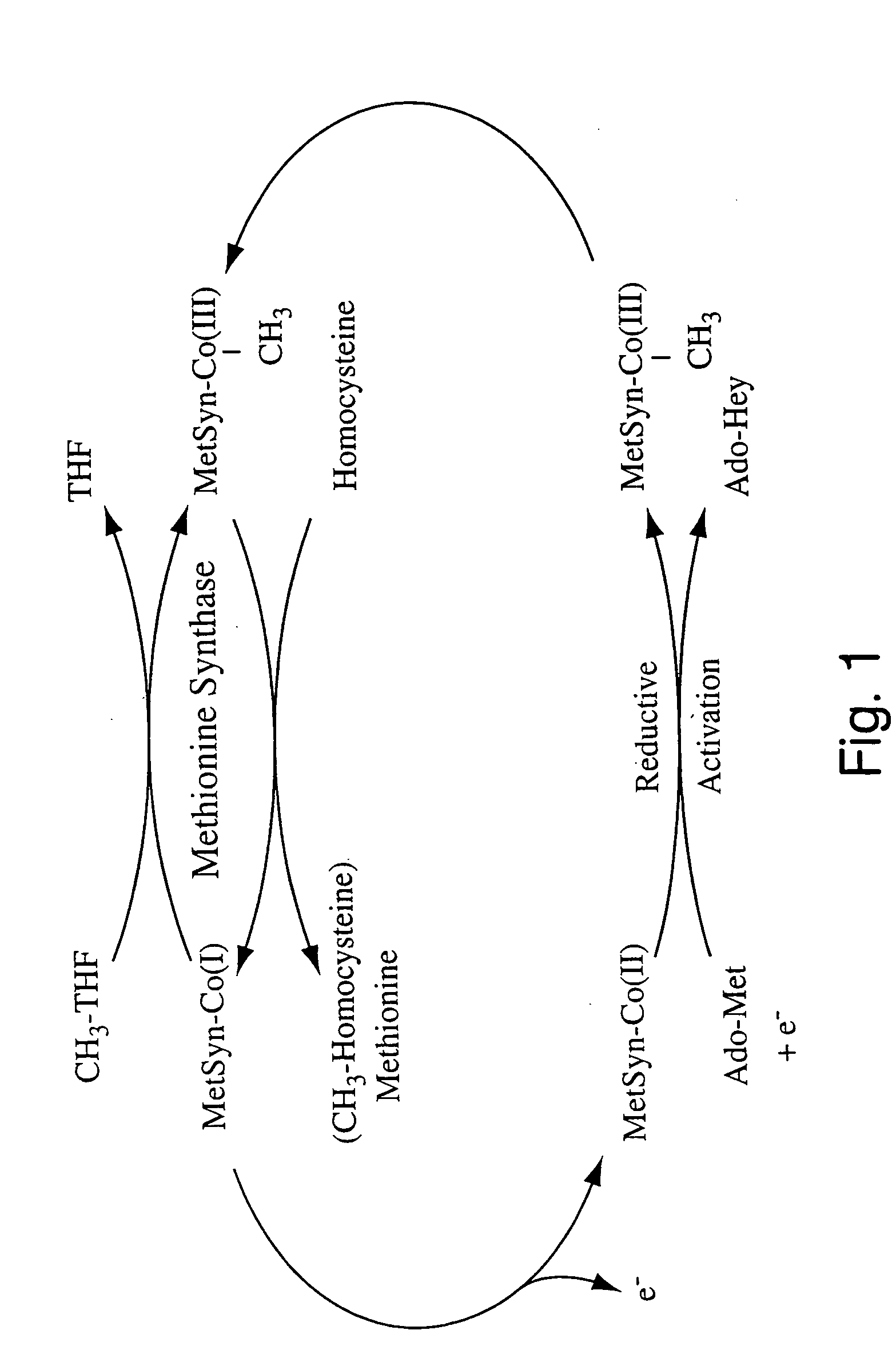
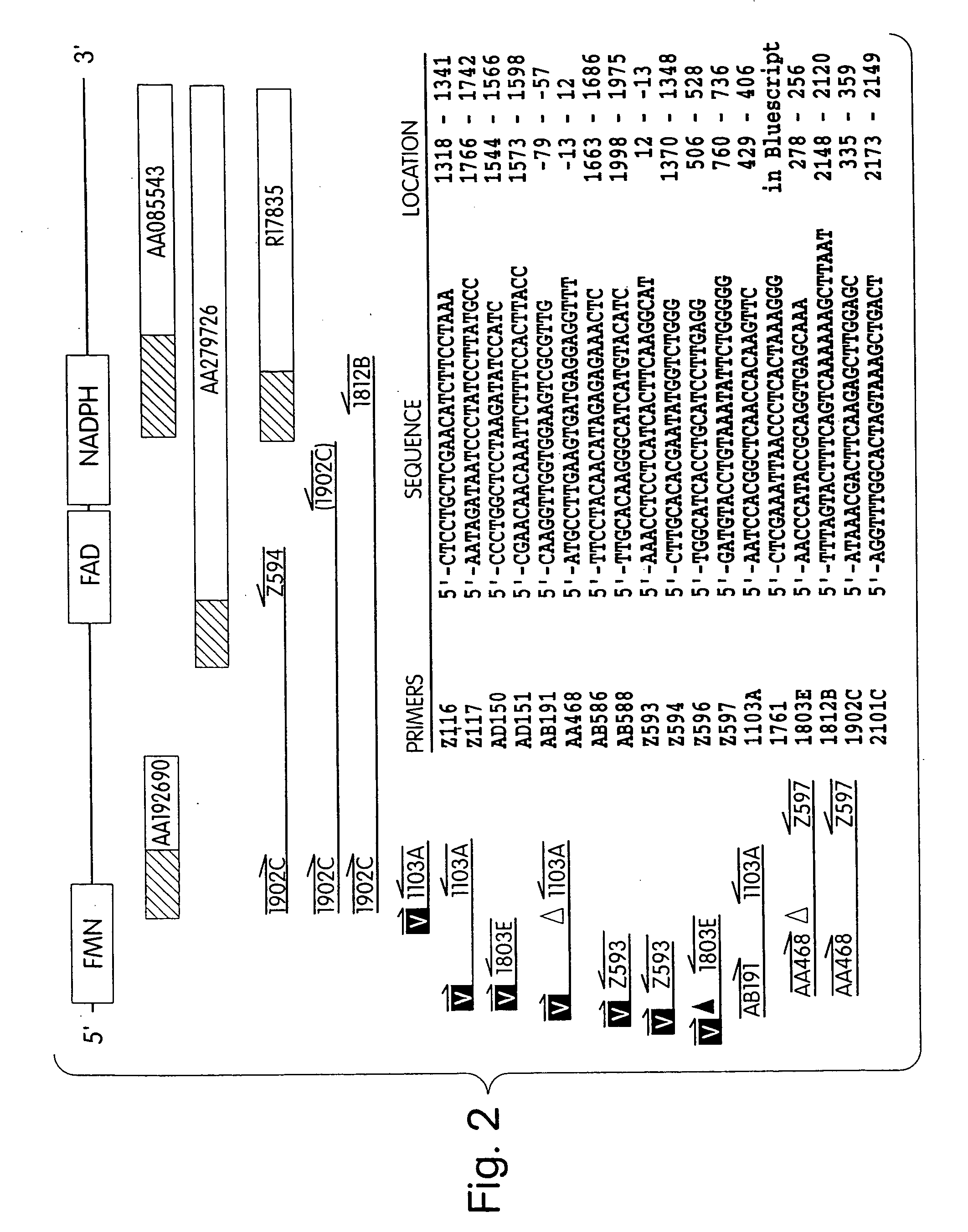
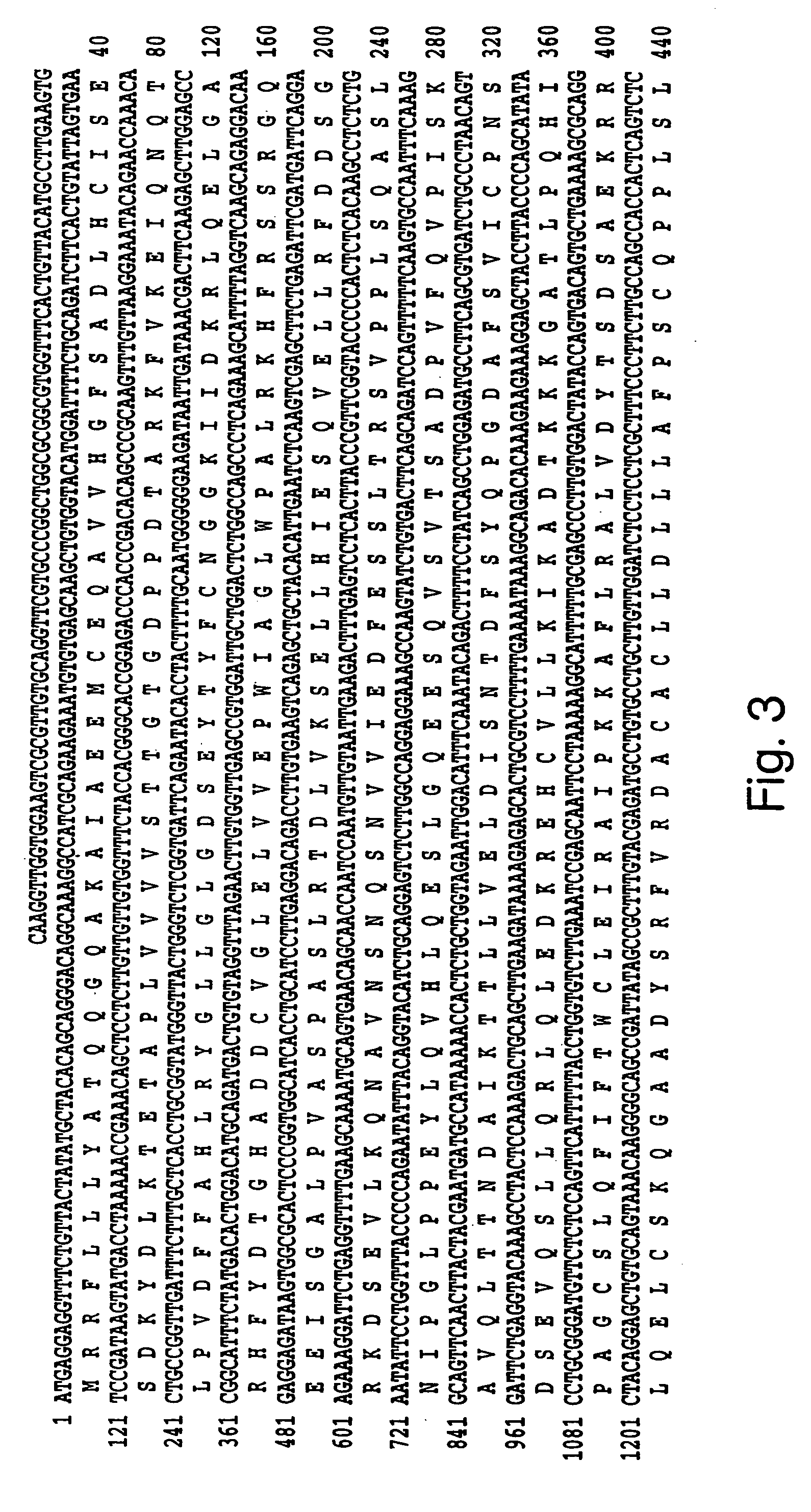
Human methionine synthase reductase: cloning, and methods for evaluating risk of neural tube deffects, cardiovascular disease, cancer and Down's Syndrome
a technology of human methionine synthase and reductase, which is applied in the direction of biochemistry apparatus and processes, enzymes, roofs, etc., can solve the problems of cardiac disease, neural tube defects, cancer, etc., and achieve the effect of increasing or decreasing the stability of mrna or polypeptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Methods
Materials
[0130] Radiolabeled compounds were from DuPont (Wilmington, Del.). A human multiple tissue Northern blot and β-actin probe were from Clontech (Palo Alto, Calif.). The random-primed DNA labelling kit was from Boehringer Mannheim (Indianapolis, Ind.). The T / A cloning kit was from Invitrogen (Carlsbad, Calif.), the Geneclean III kit was obtained from Bio101 Inc. (Vista, Calif.), and the Wizard Mini-Preps were from Promega (Madison, Wis.). Taq polymerase, AMV reverse transcriptase, Trizol reagent, and were purchased from Gibco BRL (Gaithersburg, Md.), and restriction enzymes were purchased from Gibco BRL and New England Biolabs (Beverly, Mass.). The Sequenase kits for manual sequencing of crude PCR products or plasmids were from United States Biochemicals (Cleveland, Ohio). The oligonucleotides (SEQ ID NOs: 3-20 and 49-50) were synthesized by ACGT Corporation (Toronto, Canada) or by the Sheldon Biotechnology Centre, McGill University. The sequences of oligonuc...
example ii
Cloning of the Human Methionine Synthase Reductase cDNA
[0144] More than 20 overlapping sequences homologous to the FAD and NADPH-binding domains of cytochrome P450 reductase were identified in an initial survey of the NCBI dbEST database using TblastN. We sequenced clones 550341 (accession #AA085543), 704947 (accession #AA279726) and 31776 (accession #R17835) to confirm the sequence of this part of the cDNA. Reprobing the NCBI databases with this sequence yielded a C. elegans sequence (accession #Z35595) containing binding sites for FMN, FAD and NADPH. We then used the C. elegans sequence to reprobe the dbEST database using TblastN and identified a human sequence (accession #AA192690, clone 628497) containing a putative FMN binding site similar to the one encoded by Z35595. We designed a sense primer based on the FMN binding region of AA192690 and antisense primers corresponding to the FAD / NADPH binding regions of the methionine synthase reductase candidate and amplified a sequence...
example iii
Expression of Human Methionine Reductase mRNA
[0150] A PCR product generated with primers 1902C (SEQ ID NO: 19) and 1812B (SEQ ID NO: 18) was subcloned and used to probe a Northern blot prepared from several human tissues.
[0151]FIGS. 5A and 5B show a Northern blot analysis of methionine synthase reductase expression in human tissues, with the positions of the molecular size (kb) markers indicated at the left. The 1.8 kb probe hybridized to one predominant RNA species of 3.6 kb. Methionine synthase reductase appears to be expressed to some degree in all tissues tested and is particularly abundant in skeletal muscle. In addition to the 3.6 kb band, a 3.1 kb band and a faint 6 kb band were detected in brain mRNA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com