Methods of using beta glucan as a radioprotective agent
a radioprotective agent and beta glucan technology, applied in the direction of antibacterial agents, immunological disorders, extracellular fluid disorders, etc., can solve the problems of poor patient compliance, no consensus achieved, and not provided a convenient formulation of beta glucan, so as to enhance the regeneration of promote stem cell attachment to the injury site, and enhance the regeneration of glucan-mediated hematopoietic progenitor stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
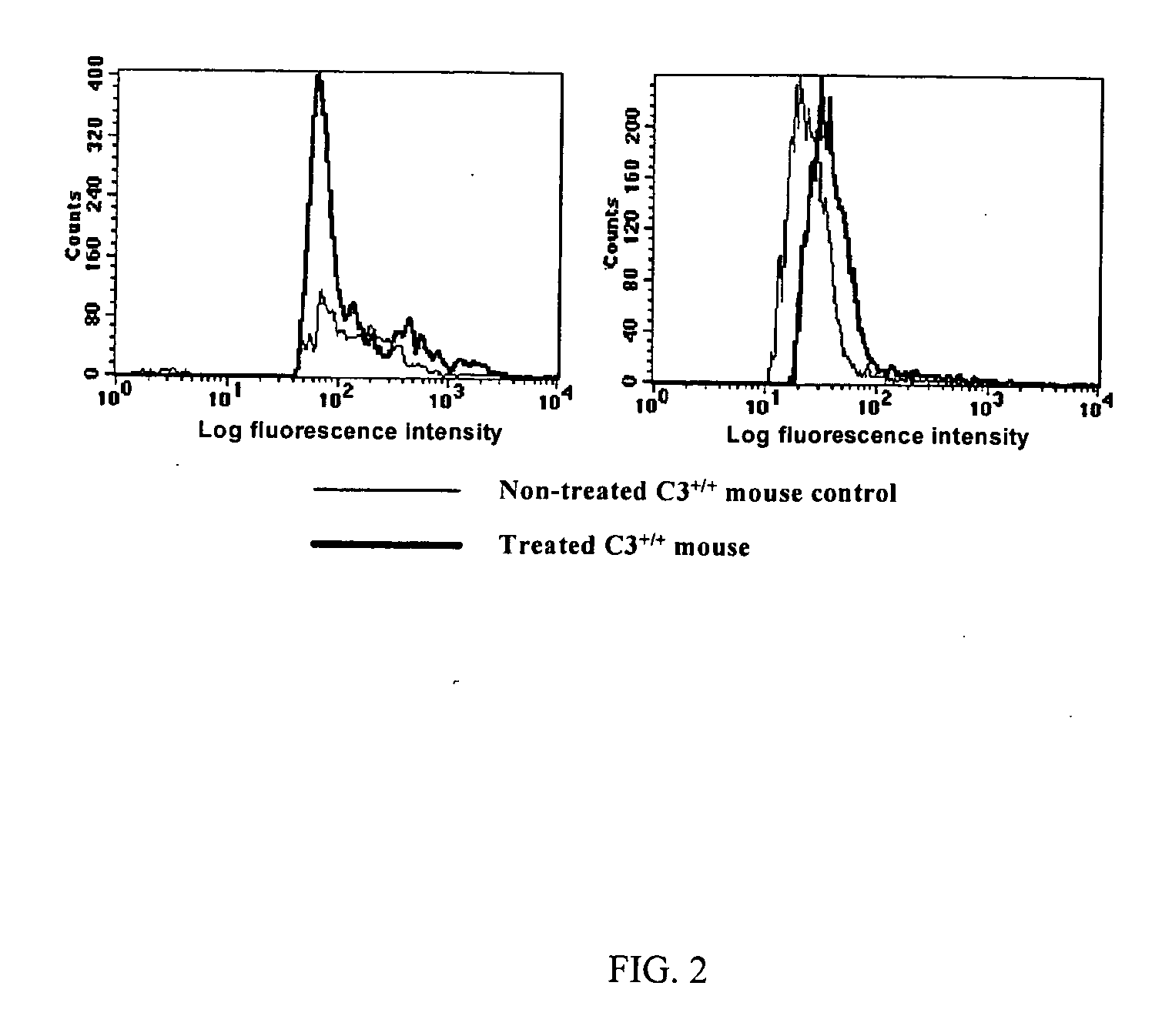
[0077] This example concerns the intravenous administration of WGP obtained using the described procedure from S. cerevisiae to mice to observe radioprotectant activity. WGP was suspended in sterile saline by placing it in a sonicating water bath at room temperature for 30 minutes to produce a fine suspension. Alternately, The WGP can be passed through a syringe needle of decreasing diameter until the material is capable of passing through the needle used for tail vein injection. A 4.0 mg / ml solution prepared in this fashion was administered to a group of 20 wild type C57Bl / 6 mice 1 day before irradiation with 6.5 Gy 60Co radiation by injecting 0.1 ml of the suspension into the tail vein. Survival rates of the treated mice changed dramatically over days 10 to 15 post-irradiation. Thirty days post-irradiation, 51% of the glucan treated mice were alive, while in control experiments, none of the mice survived beyond 21 days post-irradiation.
example 2
[0078] This example concerns the oral administration of WGP obtained using the described procedure from S. cerevisiae to mice to observe radioprotectant activity. WGP, was suspended in sterile saline by passing it through a gavage needle to ensure the elimination of lumps of material. A 0.8 mg / ml suspension prepared in this fashion was administered to a group of 20 wild type C57Bl / 6 mice 1 day before irradiation with 6.5 Gy 60Co radiation by gavaging the suspension. Dosing was continued ever day up to 10 days after irradiation. Survival rates of the treated mice were the same as that observed with intravenous administration. Thirty days post-irradiation, 51% of the glucan treated mice were alive, while in control experiments, none of the mice survived beyond 21 days post-irradiation.
example 3
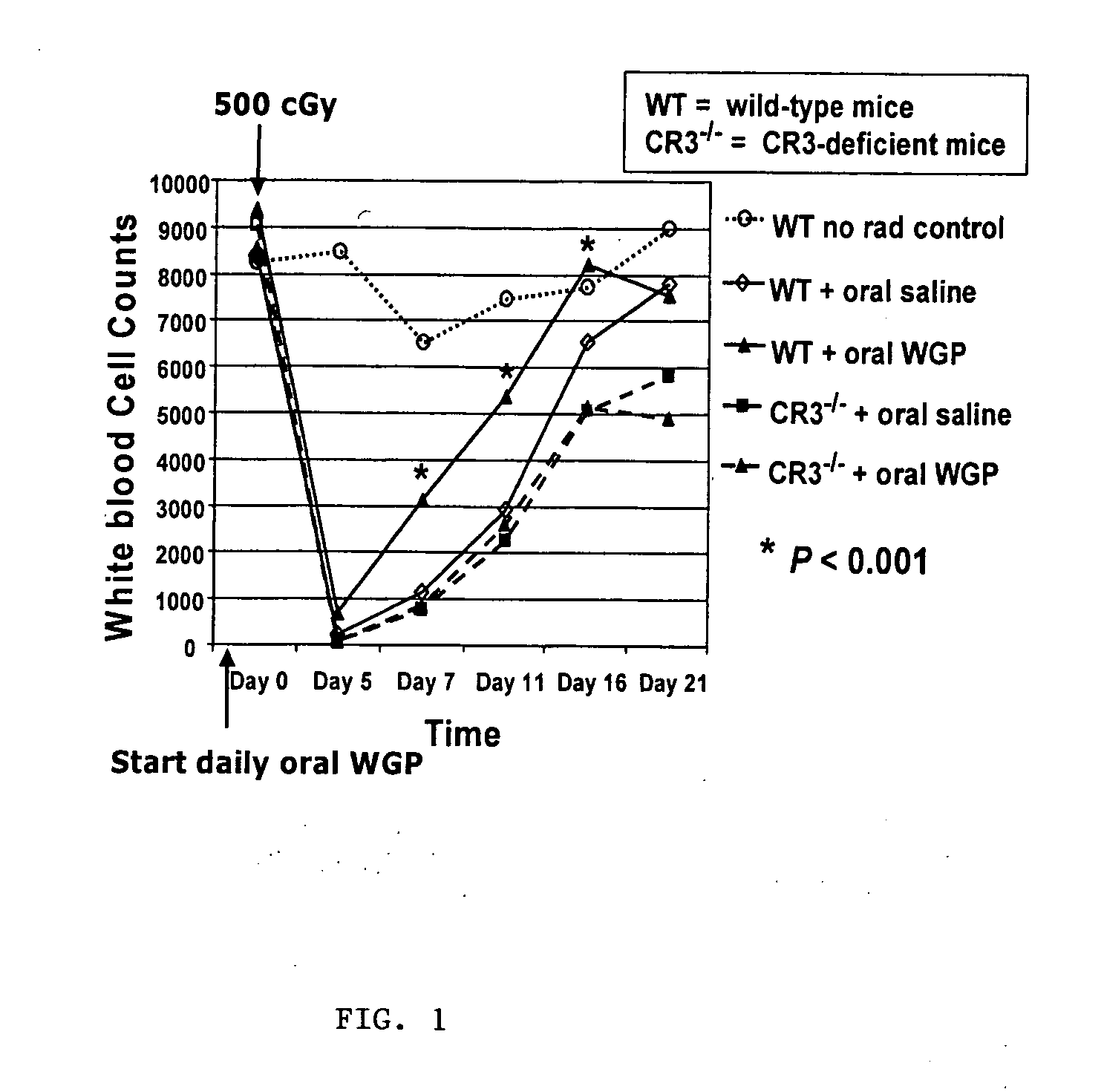
[0079] This example concerns the intravenous administration of WGP obtained using the described procedure from S. cerevisiae to mice to observe the stimulation of post-radiation myelosuppressive recovery. WGP was suspended in sterile saline by placing it in a sonicating water bath at room temperature for 30 minutes to produce a fine suspension. Alternately, The WGP can be passed through a syringe needle of decreasing diameter until the material is capable of passing through the needle used for tail vein injection. A 4.0 mg / ml solution prepared in this fashion was administered to a group of 5 wild type C57Bl / 6 mice 1 day before irradiation with a nonfatal 650-rad dose of total-body gamma radiation by injecting 0.1 ml of the suspension into the tail vein. Myelosuppression recovery, as indicated by white blood cell (WBC) count, had diverged 7 days post-irradiation, as shown on the chart below, with mice treated with WGP showing significantly higher white blood cell counts than mice tre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com