Method for the prophylaxis and/or treatment of medical disorders
a medical disorder and prophylaxis technology, applied in the field of medical disorders, can solve the problems of affecting a significant proportion of the population, and not being completely effective, and being free of adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0118] The differentiated human keratinocyte cell line, HaCaT [9] was used in the in vitro assay. Cells at passage numbers 33 to 36 were maintained as monolayer cultures in 5% V / V CO2 at 37° C. in Keratinocyte-SFM (Gibco) containing EGF and bovine pituitary extract as supplied. Media containing foetal calf serum were avoided because of the high content of IGF-I binding proteins in serum.
[0119] Feeder layer plates of lethally irradiated 3T3 fibroblasts were prepared exactly as described by Rheinwald and Green [10].
example 2
[0120] Cells were grown to 4 days post confluence in 2 cm2 wells with daily medium changes of Keratinocyte-SFM, then the medium was changed to DMEM (Cytosystems, Australia), with the following additions: 25 mM Hepes, 0.19% w / v, sodium bicarbonate, 0.03% w / v glutamine (Sigma Chemical Co, USA), 50IU / ml penicillin and 50 μg / ml streptomycin (Flow Laboratories). After 24 hours, IGF-I or tIGF-I was added to triplicate wells, at the concentrations indicated, in 0.5 ml fresh DMEM containing 0.02% v / v bovine serum albumin (Sigma molecular biology grade) and incubated for a further 21 hours. [3H]-Thymidine (0.1 μCi / well) was then added and the cells incubated for a further 3 hours. The medium was then aspirated and the cells washed once with ice-cold PBS and twice with ice-cold 10% v / v TCA. The TCA-precipitated monolayers were then solubilized with 0.25M NaOH (200 μl / well), transferred to scintillation vials and radioactivity determined by liquid scintillation counting (Pharmacia Wallac 1410 ...
example 3
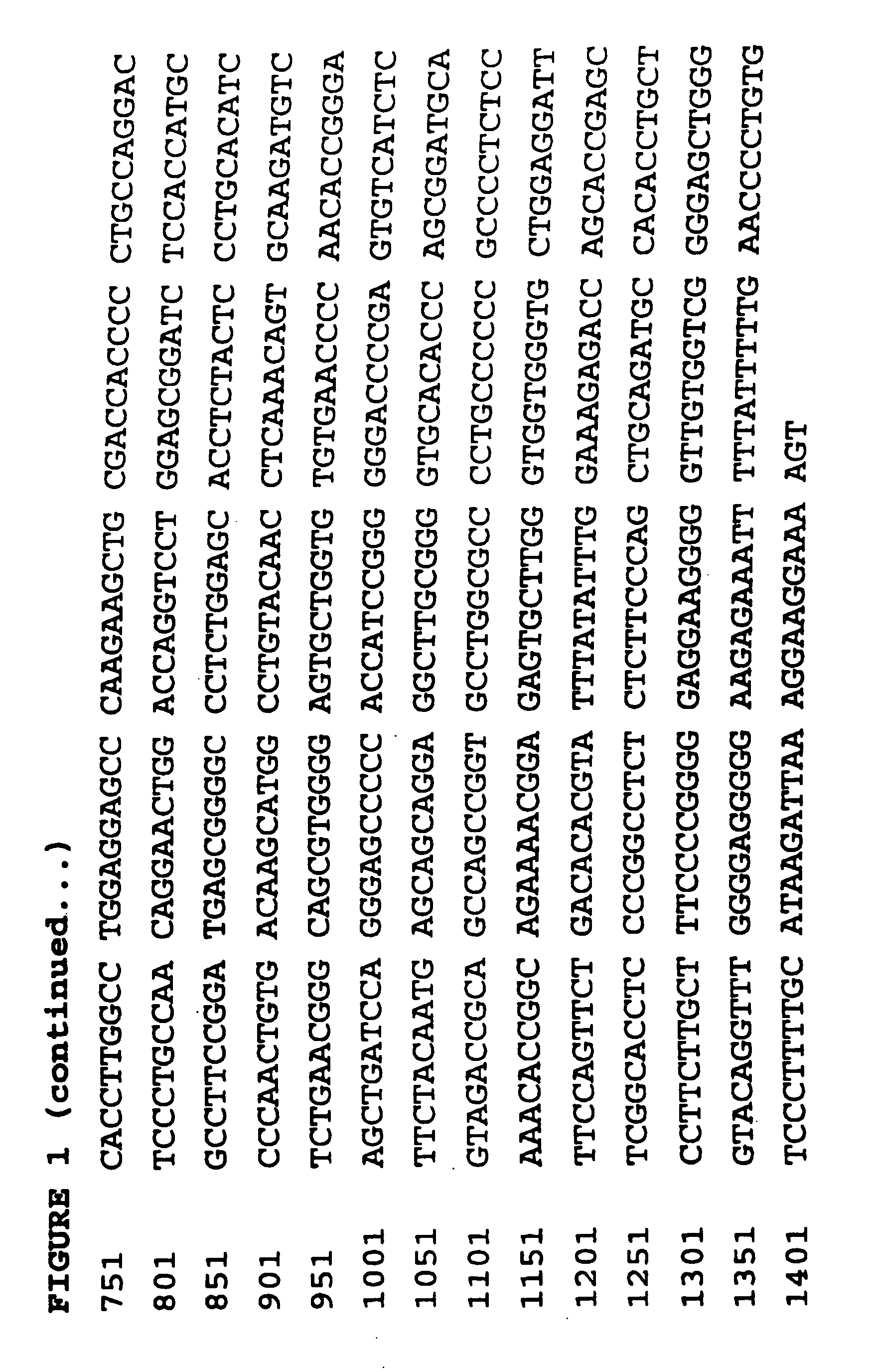
[0121] HaCaT conditioned medium (250 μl) was concentrated by adding 750 μl cold ethanol, incubating at −20° C. for 2 hours and centrifuging at 16,000 g for 20 min at 4° C. The resulting pellet was air dried, resuspended thoroughly in non-reducing Laemmli sample buffer, heated to 90° C. for 5 minutes and separated on 12% w / v SDS-PAGE according to the method of Laemmli (1970).
[0122] Separated proteins were electrophoretically transferred to nitrocellulose membrane (0.45 mm, Schleicher and Schuell, Dassel, Germany) in a buffer containing 25 mM Tris, 192 mM glycine and 20% v / v methanol. IGFBPs were then visualised by the procedure of Hossenlopp et al [11], using [125I]-IGF-I, followed by autoradiography. Autoradiographs were scanned in a BioRad Model GS-670 Imaging Densitometer and band densities were determined using the Molecular Analyst program.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| min-width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com