Method of increasing bone toughness and stiffness and reducing fractures
a technology of toughness and stiffness and bone, applied in the direction of pharmaceutical active ingredients, peptide/protein ingredients, drug compositions, etc., can solve the problems of reducing the strength of the bone, and reducing the risk of fracture, so as to increase the toughness or stiffness of bone, and reduce the incidence and/or severity of fractures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Increased Bone Strength and Density Upon Administration of rhPTH(1-34) to Rabbits Experimental Procedures
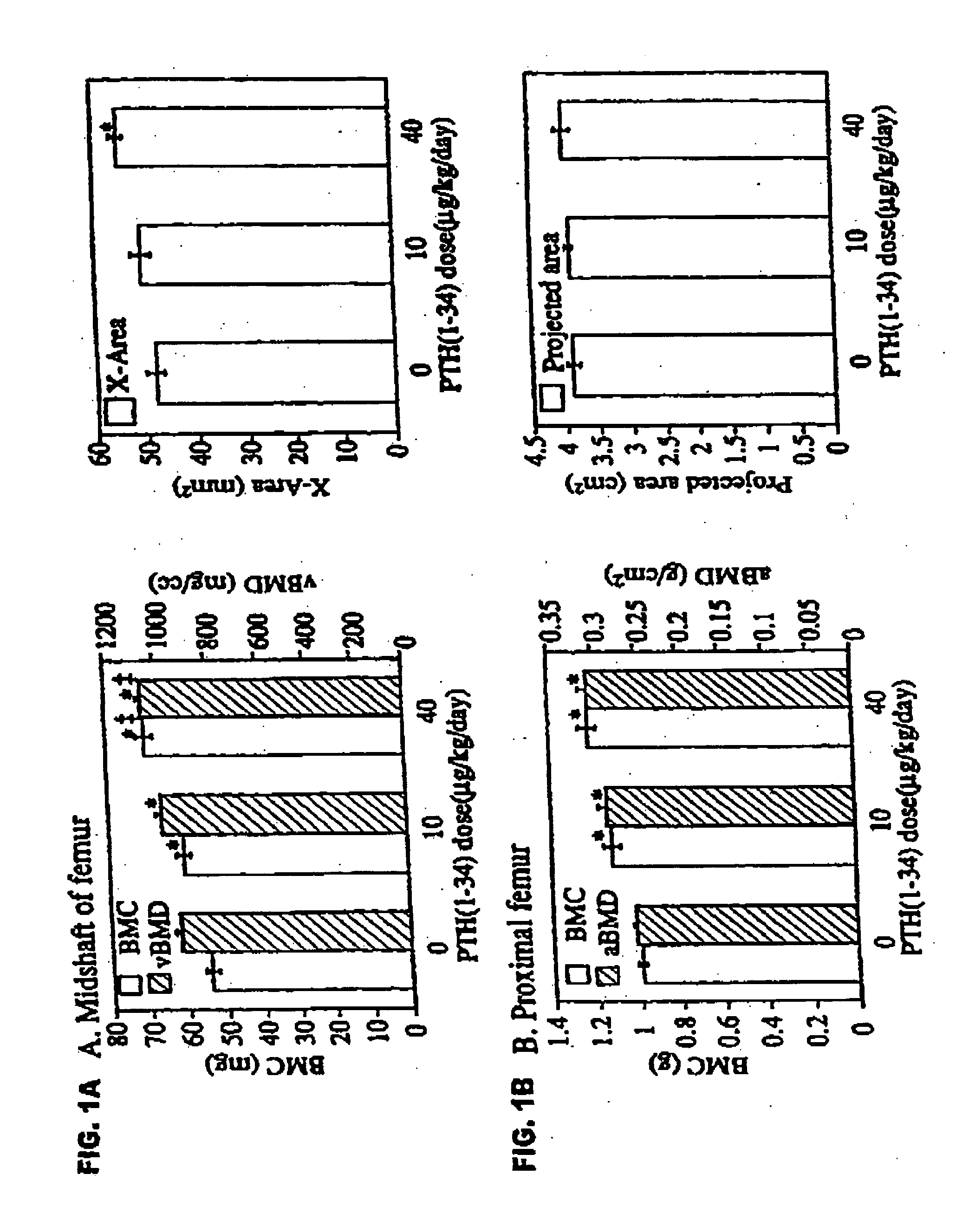
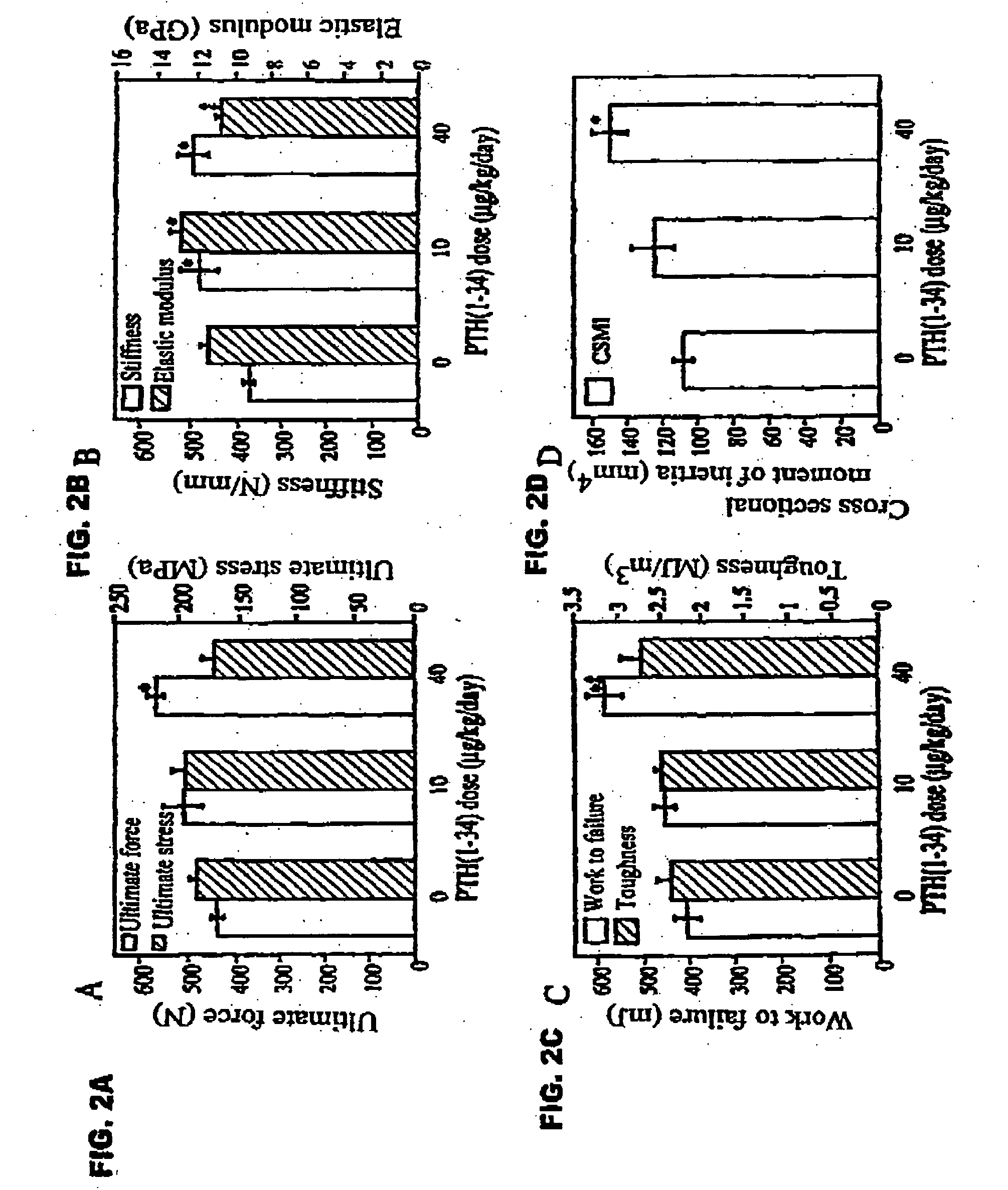
[0051] Female intact New Zealand white rabbits (HRP Inc. Denver, Pa.), one of the smallest animals that form osteons by intracortical remodeling, approximately 9 months old and weighing 3.25-3.75 kg, were sorted by mean group body weight into 3 groups of 6 animals each. Two experimental groups received biosynthetic PTH(1-34) at doses of 10 or 40 μg / ml / kg / day. The control group was given 1.0 ml / kg / day of acidified 0.9M saline containing 2% heat-inactivated rabbit sera. PTH(1-34) or vehicle were injected by once daily subcutaneous injections on 5 days a week for 140 days. Rabbits were fed rabbit lab chow containing 0.5% Ca and 0.41% P, and given water ad libitum.
[0052] The selection of doses was based on a series of preliminary studies showing that (1) after a single injection of PTH(1-34) at 100 μg / kg, serum calcium increased and failed to return to baseline by 24 hours, whereas...
example 2
Increased Bone Strength and Density Upon Administration of rhPTH(1-34) to Monkeys
Experimental Procedures
General
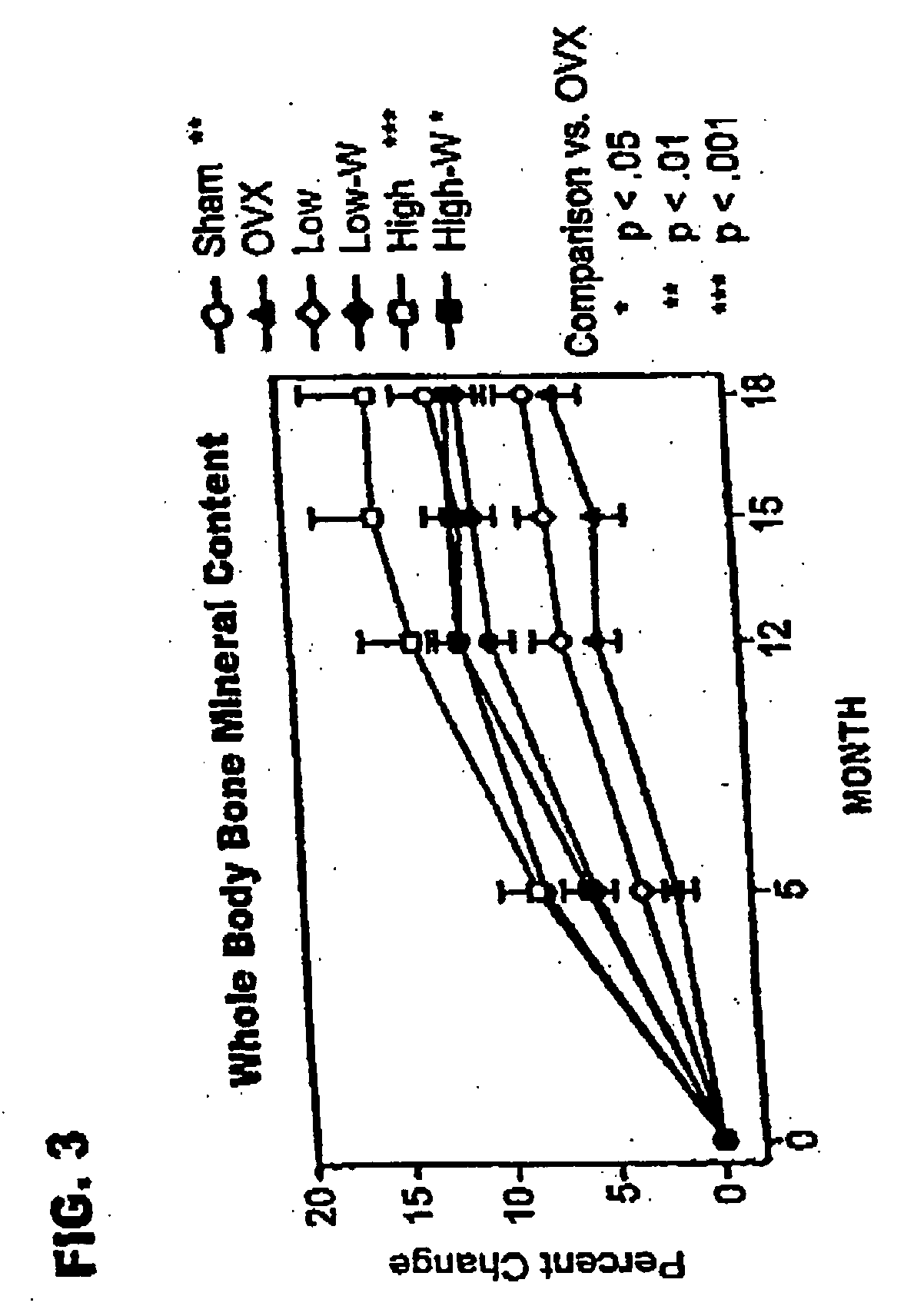
[0092] The live phase of the study used feral, adult (closed growth plates) cynomolgus primates (Macaca fascicularis), weighing 2.77±0.03 kg (mean±standard error of the mean [SEM]). Monkeys were held in quarantine for 3 months, then started on a diet containing 0.3% calcium, 0.3% phosphate, and 250 IU vitamin D3 / 100 g diet, and given fluoridated water (I ppm fluoride) ad libitum. The calcium content corresponded to 1734 mg calcium / 2000 calories. After 1 month on the diet, animals were sorted into groups of 21 or 22, sham operated or ovariectomized. Once daily subcutaneous injections of vehicle (sham and ovariectomized controls) or rhPTH(1-34), at 1 μg / kg (PTH1) or 5 μg / kg (PTH5), were started 24 hours after ovariectomy. Animals were treated for either 18 months (PTH1 and PTH5), or for 12 months followed by withdrawal of treatment (PTH1-W and PTH5-W).
[0093] The study g...
example 3
Increased Bone Strength and Density, and Reduced Fractures Upon Administration of rbPTH(1-34) to Humans
Number of Subjects:
[0137] rhPTH(1-34): 1093 enrolled, 848 finished. [0138] Placebo: 544 enrolled, 447 finished.
Diagnosis and Inclusion Criteria: [0139] Women ages 30 to 85 years, postmenopausal for a minimum of 5 years, with a minimum of one moderate or two mild atraumatic vertebral fractures.
Dosage and Administration:
Test Product (Blinded) [0140] rhPTH(1-34): 20 μg / day, given subcutaneously [0141] rhPTH(1-34): 40 μg / day, given subcutaneously
Reference Therapy (Blinded) [0142] Placebo study material for injection
Duration of Treatment: [0143] rhPTH(1-34): 17-23 months (excluding 6-month run-in phase) [0144] Placebo: 17-23 months (excluding 6-month run-in phase)
Criteria for Evaluation:
[0145] Spine x-ray; serum biological markers (calcium, bone-specific alkaline phosphatase, procollagen 1 carboxy-terminal propeptide); urine markers (calcium, N-telopeptide, free deoxypyr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com