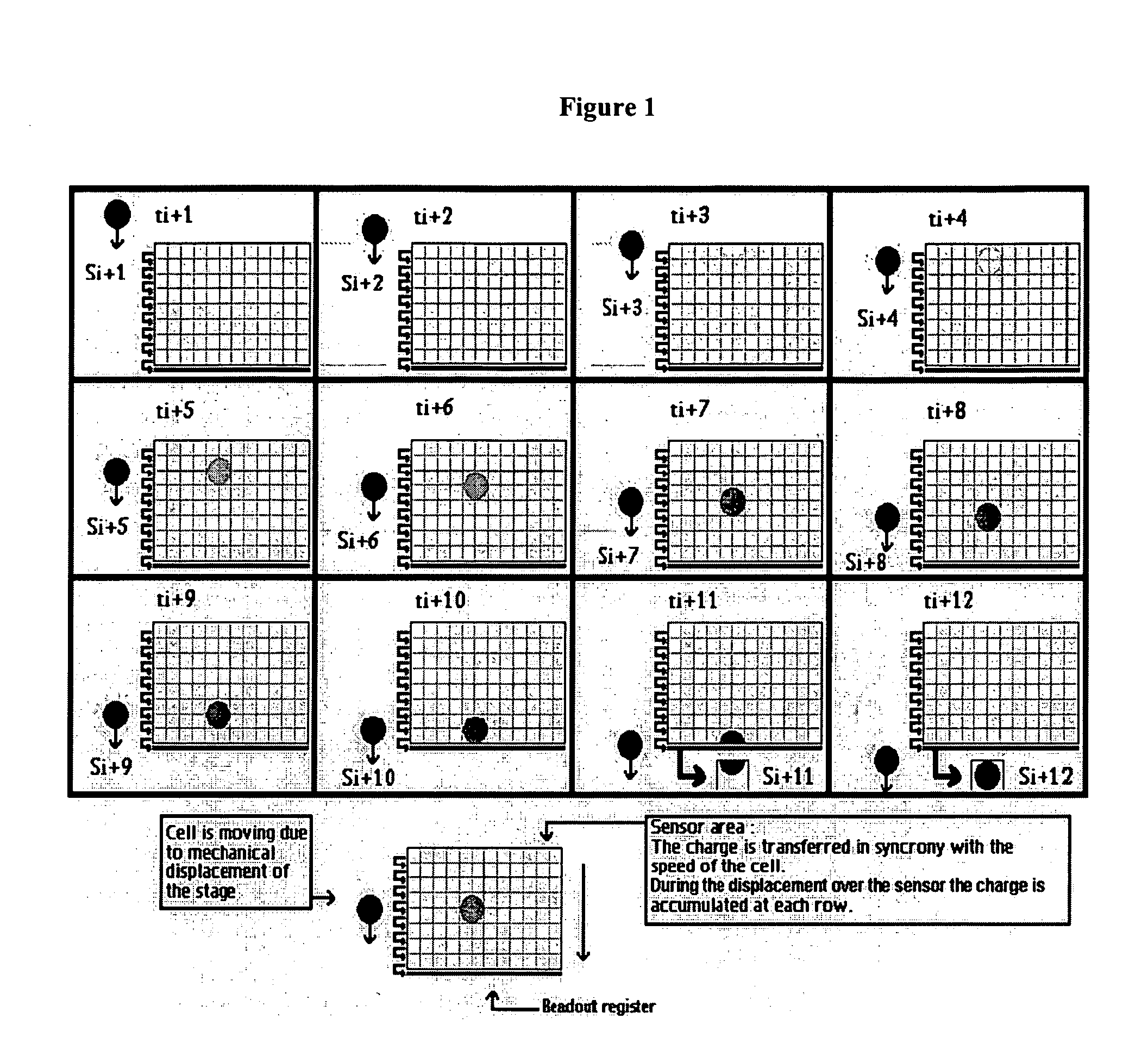
Devices and methods to image objects by time delay integration
a technology of time delay and image objects, applied in the field of devices and methods to image objects by time delay integration, can solve the problems of insufficient sensitivity of other currently available methodologies for accurate classification and typing of rare events such as circulating tumor cells, and lack of evidence, so as to improve detection, enumeration and classification, and avoid cell loss. , the effect of high quality fluorescence images
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
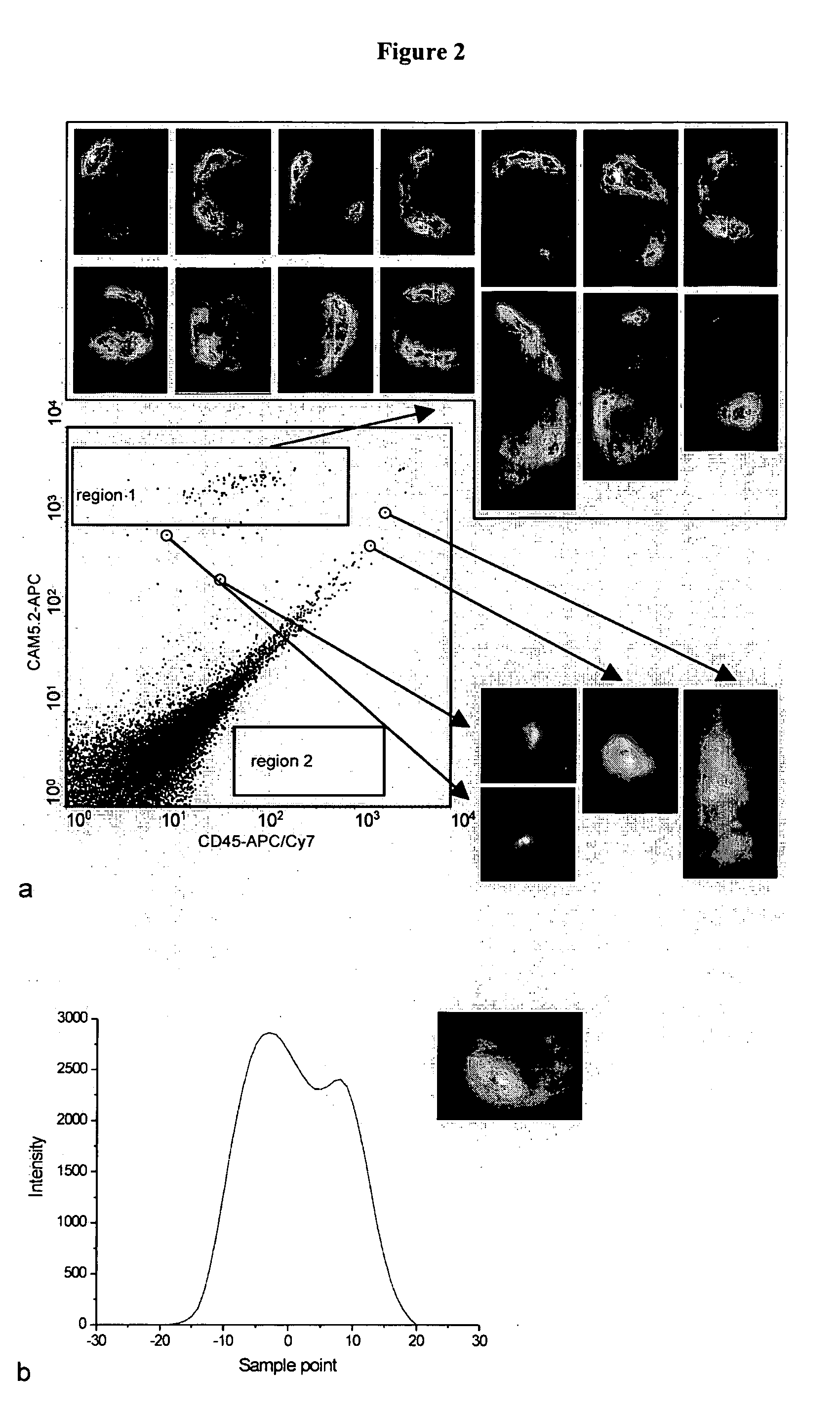
[0077] For these experiments, 10 μl of fixed SKBR3 cells (50,000 cells / ml) were mixed with 290 μl of EDTA blood. Also added at the same time were 100 μl of magnetic ferrofluid coated with anti-EpCAM (magnetic particles of about 200 nm size coated with proteins, streptavidin and biotinylated EpCAM antibody), an antibody specific for epithelial cells and known to be present on SKBR3 cells (cultured at Immunicon Corp., Huntingdon Valley, Pa.), 10 μl of allophycocyanin (APC) conjugated to monoclonal antibodies recognizing anti-cytokeratin species or cytoskeletal proteins present in epithelial cells (e.g. SKBR3 cells that are epithelium derived) and 10 μl CD45-APC / Cy7 (Caltag, Burlingame, Calif.) to identify leukocytes and identify leukocytes that may nonspecifically bind to cytokeratin antibody. After 15 minutes incubation, 50 μl of this blood reaction mixture was injected into the chamber. The chamber was placed in the Cell Tracks magnet assembly and after two minutes collection time, ...
example 2
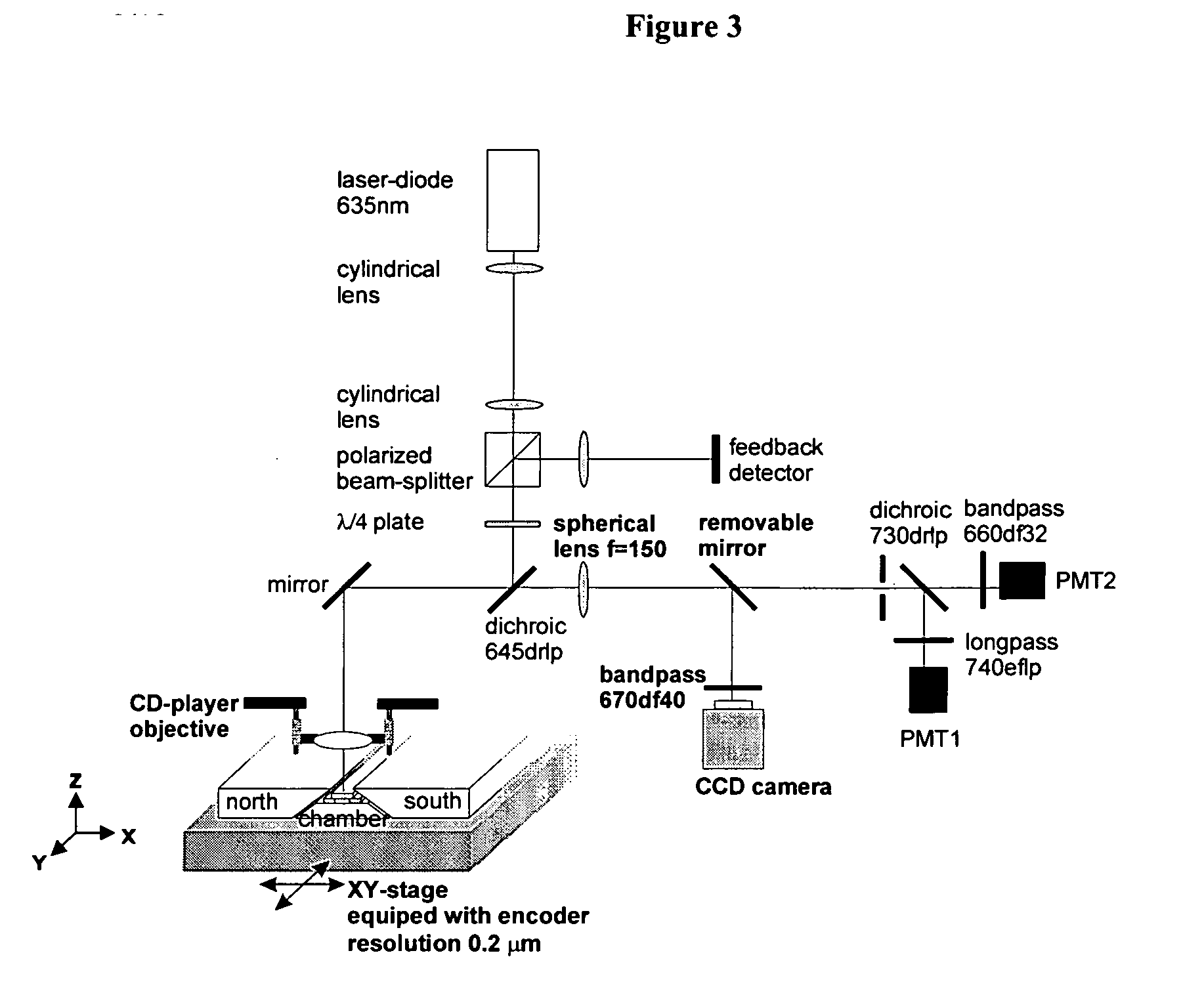
[0078] In this experiment 100 μl of EDTA anti-coagulated blood, 50 μl of ferrofluid containing 5 μg of CD45-labeled ferromagnetic nanoparticles, 1.5 μl CD4-APC and 25 μl of 10−5 M Oxazine 750 perchlorate (Exciton Inc., Dayton, Ohio) were added. The optimum concentration of the reagents was obtained by serial titration of each of the reagents. After incubation for 15 minutes, 300 μl PBS was added and 50 μl of the blood mixture was placed into the capillary that was already placed between the magnets. The capillary has a glass bottom shaped in a way that it fits between the 70° tilted faces of the magnets. Two strips of double-sided tape with a thickness of 0.5 mm (3M Co., St. Paul, Minn.) were placed on the glass with spacing of 3 mm to form the sidewalls of the capillary. Ni lines, about 30 μm wide and about 0.2 μm thick, were produced by standard photolithographic techniques on a 7740 Pyrex® glass wafer (Corning International, Germany). Wafers were cut in pieces of 4 mm×25 mm and t...
example 3
[0079] The experiment described in example 2 was repeated but time resolved images were taken with the CellTracks imaging system from Oxazine 750 stained and CD45 ferrofluid captured leukocytes in whole blood. FIG. 15 shows four examples of images taken at 20 seconds intervals. The distribution of the fluorescence within the cells is clearly changing between the time intervals and different cells behave differently as is obvious from the cells followed in frame 3 and 4. In both frames images from two cells in close proximity are taken and the differences in uptake and cellular distribution of the Oxazine 750 are apparent. From these examples it is obvious that this imaging system has a unique capability to perform functional analysis of cells as, for example, one can study the responses of cells in blood to drugs or other components in real time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solid angle | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| time span | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com