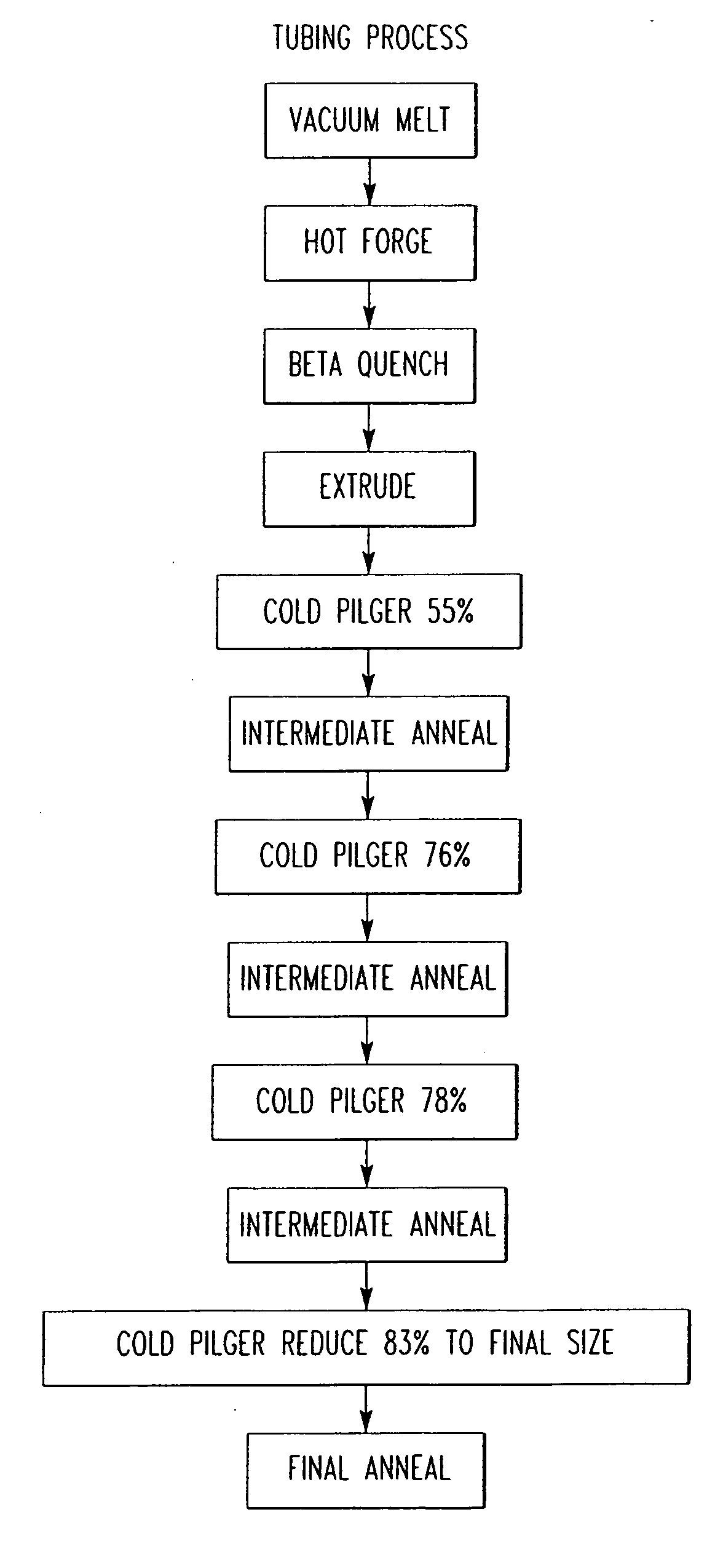
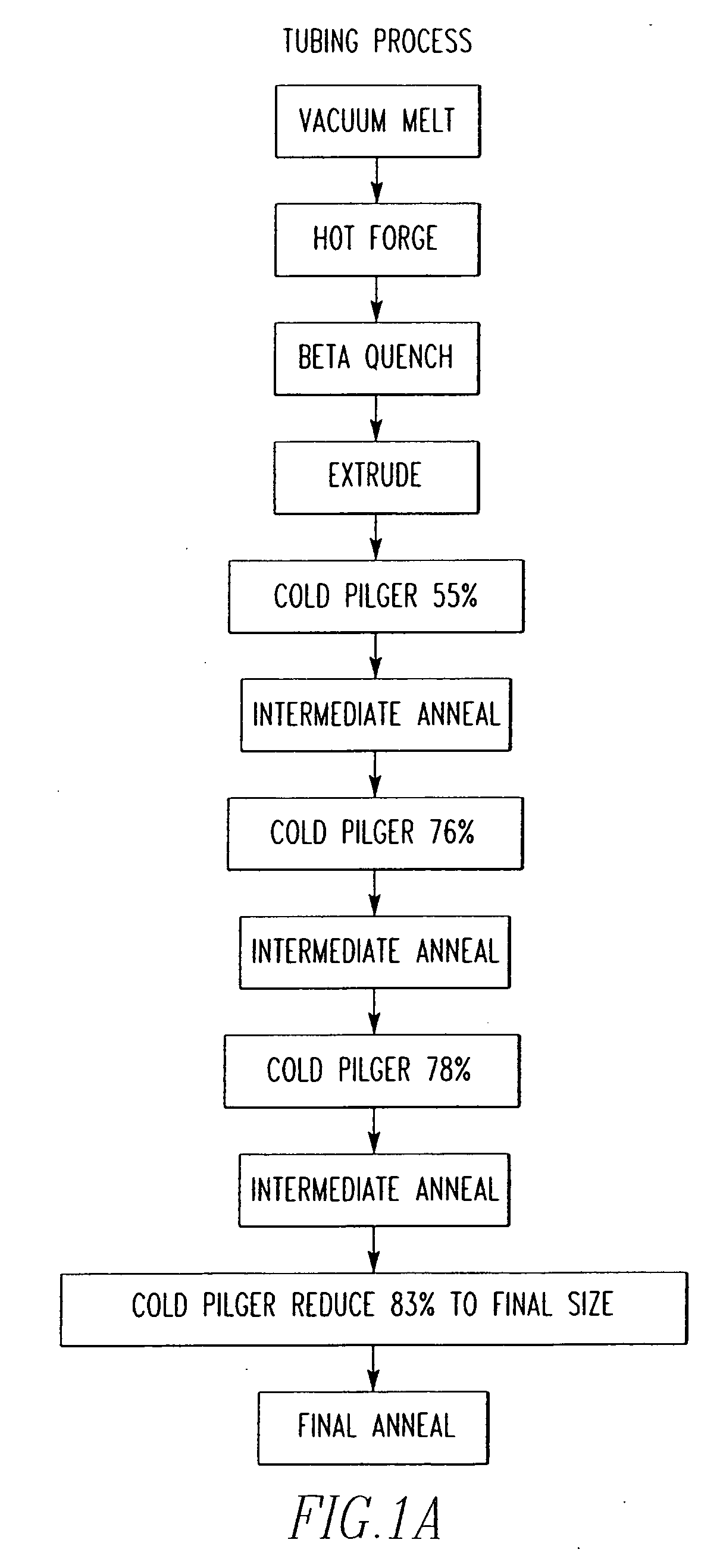
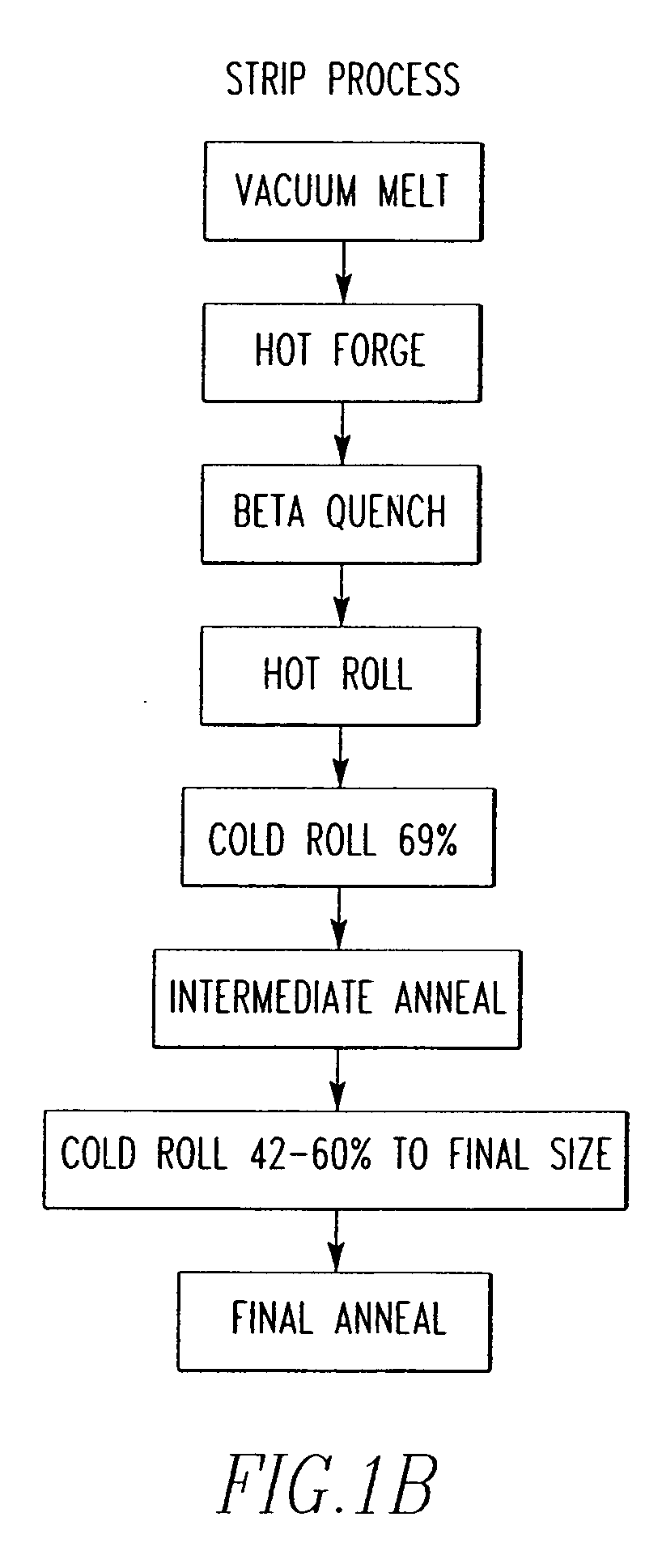
Zirconium alloys with improved corrosion resistance and method for fabricating zirconium alloys with improved corrosion
a zirconium alloy and corrosion resistance technology, applied in the field of zirconium based alloys, can solve the problems of increasing alloy corrosion, increasing alloy corrosion, and increasing the thickness of the outer layer of non-protective materials, so as to improve the corrosion resistance of welds, improve the chemistry of alloys, and improve the corrosion resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
[0049] the present invention is a zirconium alloy having, by weight percent, about 0.6-1.5% Nb; 0.05-0.4% Sn, 0.01-0.1% Fe, 0.02-0.3% Cu, 0.1-0.3% V, 0-0-0.5% Cr and at least 97% Zr including impurities, hereinafter designated as Alloy X1. This embodiment, and all subsequent embodiments, should have no more than 0.50 wt. % additional other component elements, preferably no more than 0.30 wt. % additional other component elements, such as nickel, chromium, carbon, silicon, oxygen and the like, and with the remainder Zr. Chromium is an optional addition to Alloy X1. Wherein chromium is added to Alloy X1, the alloy is hereinafter designated as Alloy X1+Cr.
[0050] A preferred composition of Alloy X1 alloy has weight percent ranges for the alloy with about 1.0% Nb; 0.3% Sn, 0.05% Fe, 0.18% V, 0.12% Cu, and at least 97% Zr. A preferred composition of Alloy X1+Cr has weight percent ranges for the alloy with about 1.0% Nb; 0.3% Sn, 0.05% Fe, 0.18% V, 0.12% Cu, 0.2% Cr and at least 97% Zr.
[0...
second embodiment
[0055] the present invention is a zirconium alloy having, by weight percent, about, about 0.6-1.5% Nb; 0.01-0.1% Fe, 0.02-0.3% Cu, 0.15-0.35% Cr and at least 97% Zr, hereinafter designated as Alloy X4. A preferred composition of Alloy X4 has weight percent ranges for the alloy with about 1.0% Nb, about 0.05% Fe, about 0.25% Cr, about 0.08% Cu, and at least 97% Zr.
[0056] The preferred Alloy X4 was fabricated into tubing and its corrosion rate was compared with the corrosion rate of Standard ZIRLO. Alloy X4 and ZIRLO were each tested for long term corrosion resistance in 680° F. water. Alloy X4, ZIRLO 1 and ZIRLO 2 tubing were placed in a long term 680° F. water autoclave test for a period of about 250 days, wherein Alloy X4 was the preferred embodiment of Alloy X4, ZIRLO 1 comprised, by weight percentage, 0.89 Nb, 0.94 Sn, 0.09 Fe, remainder Zr, and ZIRLO 2 comprised 0.97 Nb, 0.97 Sn, 0.11 Fe, remainder Zr. The tubing was measured for weight gain rates, wherein the weight gain is att...
third embodiment
[0057] the present invention is a zirconium alloy having, by weight percent, about 0.2-1.50% Nb; 0.05-0.4% Sn, 0.25-0.45% Fe, 0.15-0.35% Cr, 0.01-0.1% Ni, and at least 97% Zr, hereinafter designated as Alloy X5. This composition should have no more than 0.5 wt. % additional other component elements, preferably no more than 0.3 wt. % additional other component elements, such as carbon, silicon, oxygen and the like, and with the remainder Zr.
[0058] A preferred composition of Alloy X5 has weight percent values for the alloy with about 0.7% Nb; about 0.3% Sn. about 0.35% Fe, about 0.25% Cr, about 0.05% Ni, and at least 97% Zr. Hereinafter, this alloy will be referred to as the first embodiment of Alloy X5.
[0059] The preferred embodiment of Alloy X5 was fabricated into tubing and its corrosion rate was compared to that of a series of alloys likewise fabricated into tubing. As shown in Table 4, Alloy A, a low Nb-high Sn predecessor of Alloy X5 (U.S. Pat. No. 5,254,308 having chemical com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com