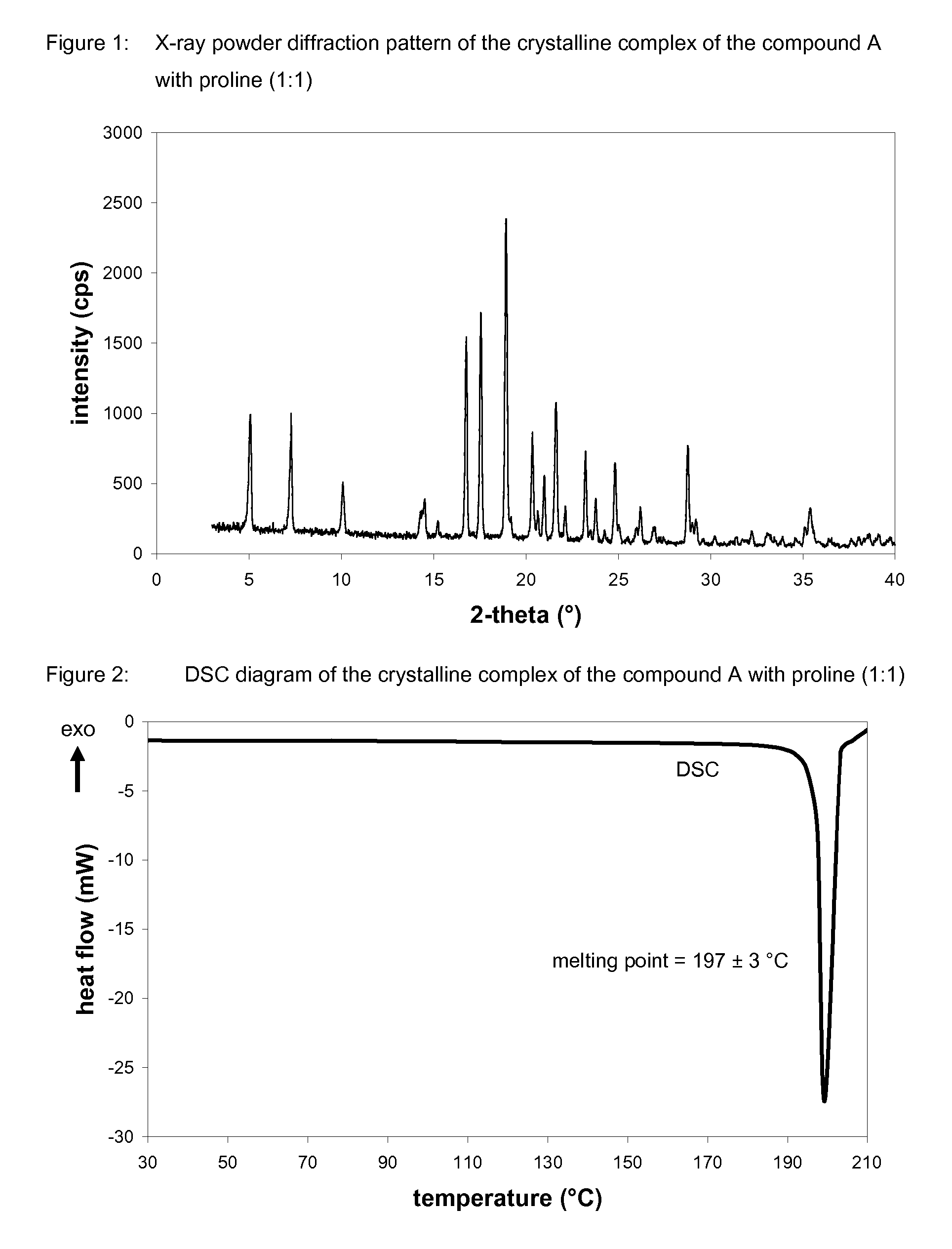
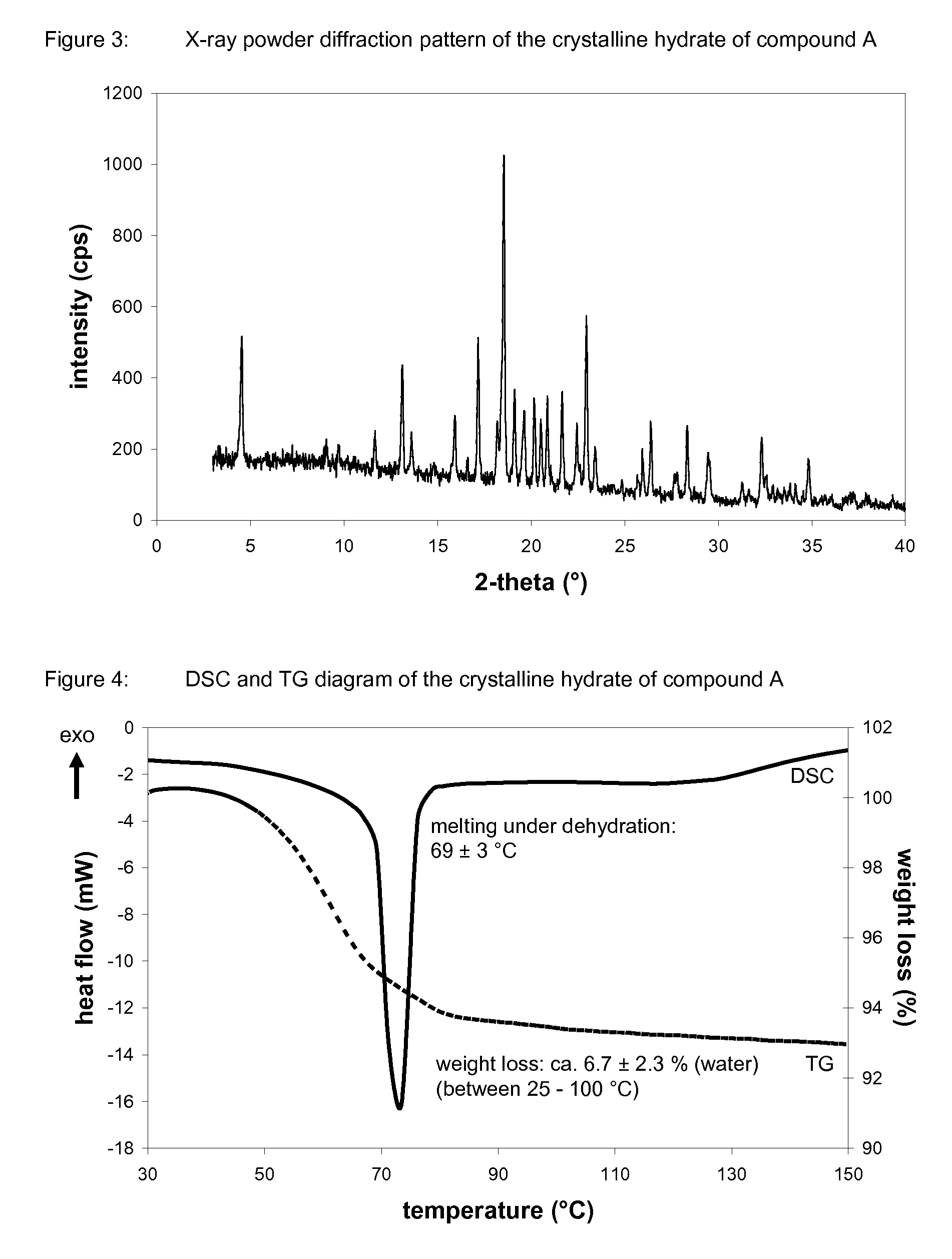
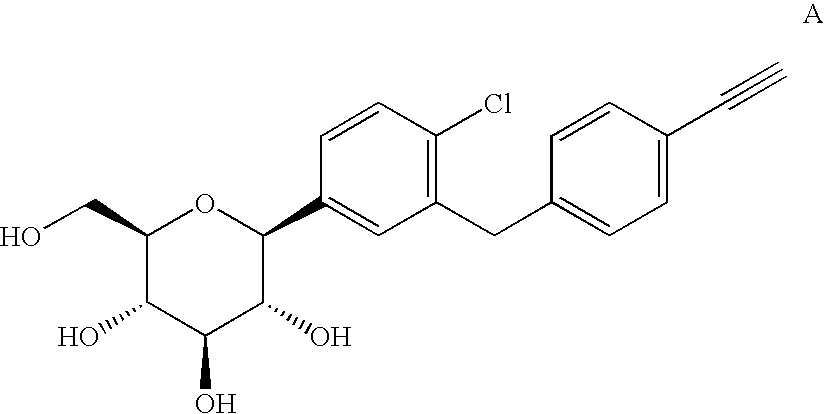
Crystalline forms of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-ethynyl-benzyl)-benzene, methods for its preparation and the use thereof for preparing medicaments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
[0100]
(5-bromo-2-chloro-phenyl)-(4-methoxy-phenyl)-methanone
[0101] 38.3 mL oxalyl chloride and 0.8 mL dimethylformamide are added to a mixture of 100 g of 5-bromo-2-chloro-benzoic acid in 500 mL dichloromethane. The reaction mixture is stirred for 14 h, then filtered and separated from all volatile constituents in the rotary evaporator. The residue is dissolved in 150 mL dichloromethane, the solution is cooled to −5° C., and 46.5 g of anisole are added. Then 51.5 g of aluminum trichloride are added batchwise so that the temperature does not exceed 5° C. The solution is stirred for another 1 h at 1-5° C. and then poured onto ice. The organic phase is separated and the aqueous phase is extracted another three times with dichloromethane. The combined organic phases are washed with aqueous 1 M hydrochloric acid, twice with 1 M sodium hydroxide solution and with brine.
[0102] Then the organic phase is dried, the solvent is removed and the residue is recrystallised from ethanol.
[0103] Y...
example ii
[0105]
4-bromo-1-chloro-2-(4-methoxy-benzyl)-benzene
[0106] A solution of 86.2 g (5-bromo-2-chloro-phenyl)-(4-methoxy-phenyl)-methanone and 101.5 mL triethylsilane in 75 mL dichloromethane and 150 mL acetonitrile is cooled to 10° C. Then with stirring 50.8 mL of boron trifluoride etherate are added so that the temperature does not exceed 20° C. The solution is stirred for 14 h at ambient temperature, before another 9 mL triethylsilane and 4.4 mL boron trifluoride etherate are added. The solution is stirred for a further 3 h at 45-50° C. and then cooled to ambient temperature. A solution of 28 g potassium hydroxide in 70 mL water is added and the mixture is stirred for 2 h. Then the organic phase is separated and the aqueous phase is extracted three times with diisopropylether. The combined organic phases are washed twice with 2 M potassium hydroxide solution and once with brine and then dried over sodium sulfate. After the solvent has been eliminated the residue is stirred in ethanol...
example iii
[0109]
4-(5-bromo-2-chloro-benzyl)-phenol
[0110] A solution of 14.8 g 4-bromo-1-chloro-2-(4-methoxy-benzyl)-benzene in 150 mL dichloromethane is cooled in an ice bath. Then 50 mL of a 1 M solution of boron tribromide in dichloromethane are added, and the solution is stirred for 2 h at ambient temperature. The solution is then cooled in an ice bath again, and saturated potassium carbonate solution is added dropwise. At ambient temperature the mixture is adjusted with aqueous 1 M hydrochloric acid to a pH of 1, the organic phase is separated and the aqueous phase is extracted another three times with ethyl acetate. The combined organic phases are dried over sodium sulfate and the solvent is removed completely.
[0111] Yield: 13.9 g (98% of theory)
[0112] Mass spectrum (ESI−): m / z=295 / 297 / 299 (Br+Cl) [M−H]−
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com