Cerium carbonate powder, method for preparing the same, cerium oxide powder made therefrom, method for preparing the same, and CMP slurry comprising the same
a technology of cerium oxide and cerium carbonate, which is applied in the field of cerium oxide powder made therefrom method for preparing same, etc., can solve the problems of difficult to prepare particles with a high crystallinity, above method is not amenable to mass production, and above method is dangerous, so as to achieve easy control of size and shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
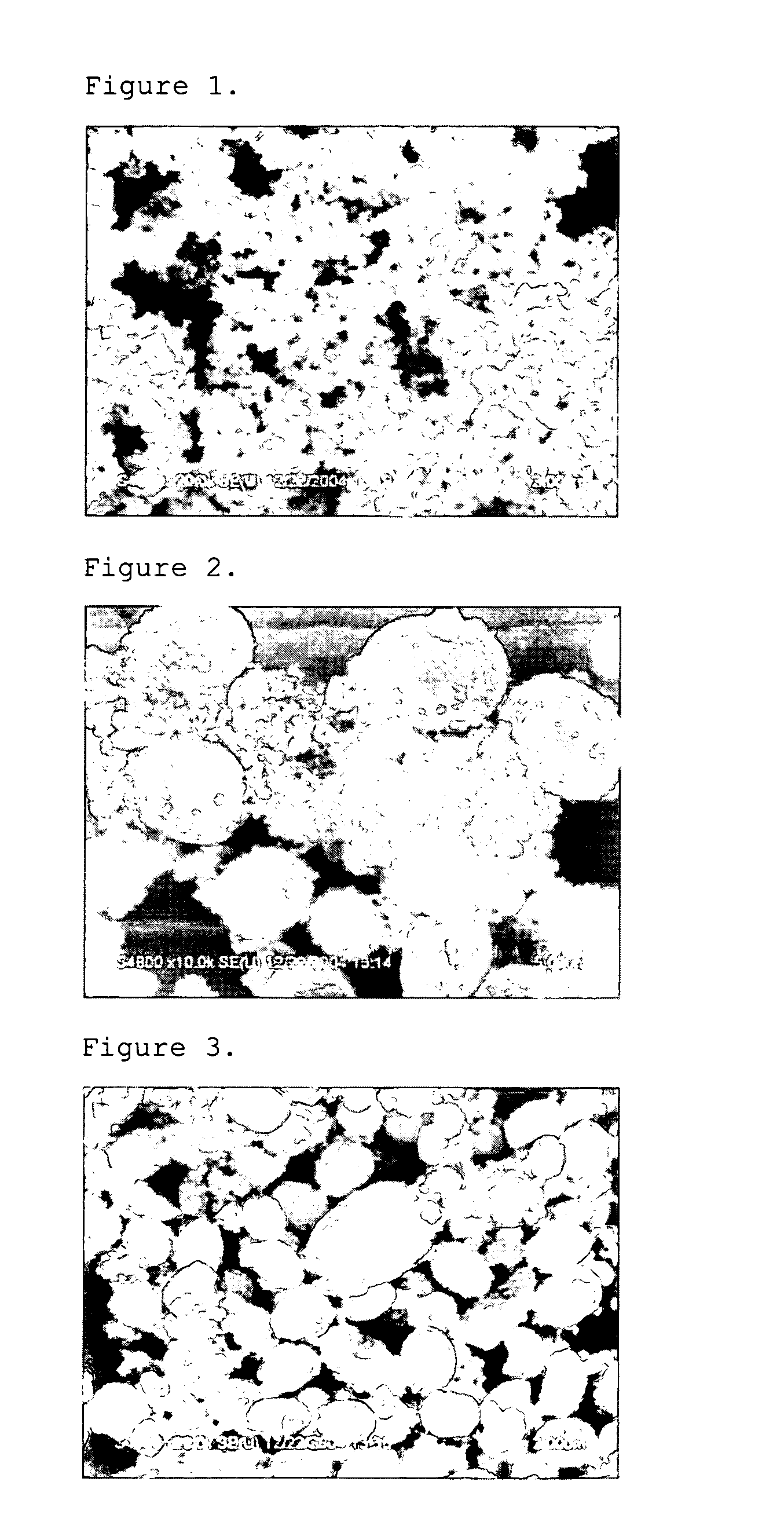
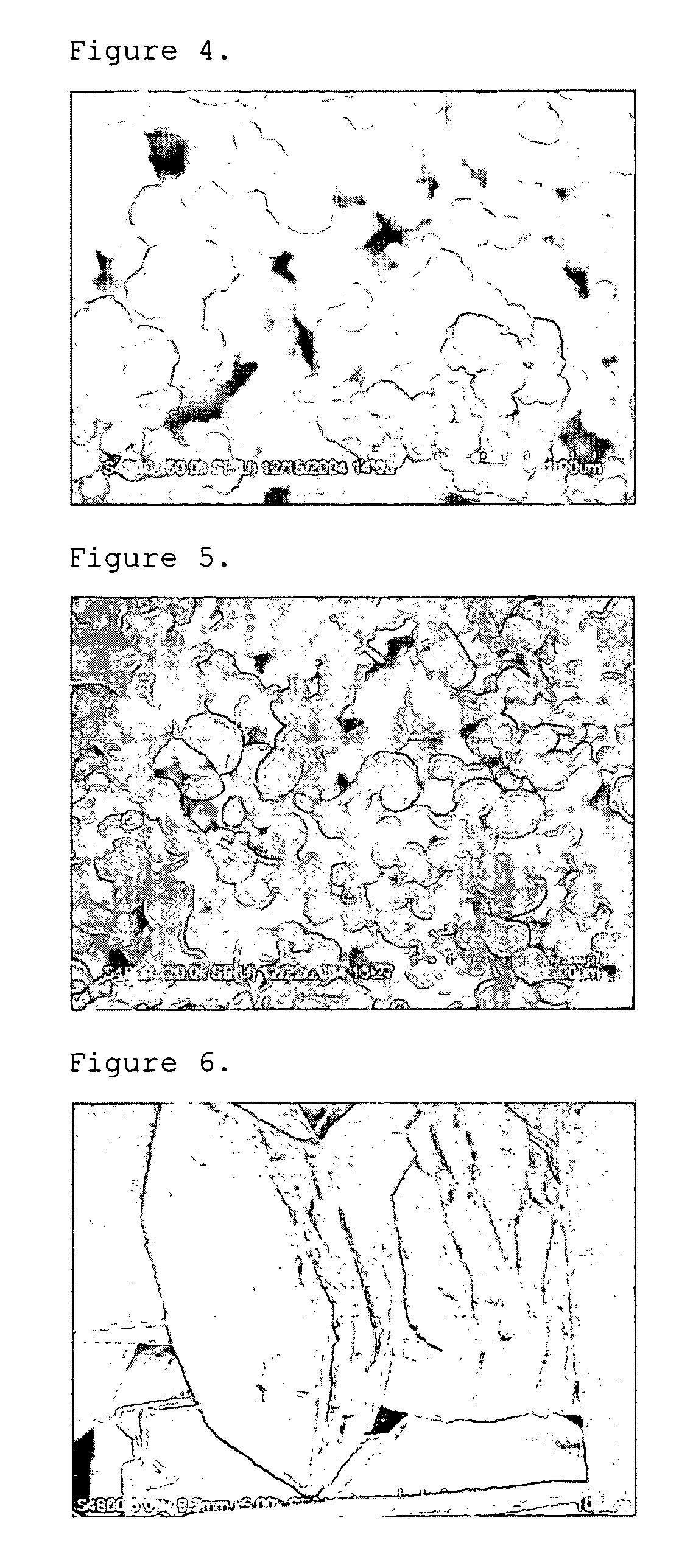
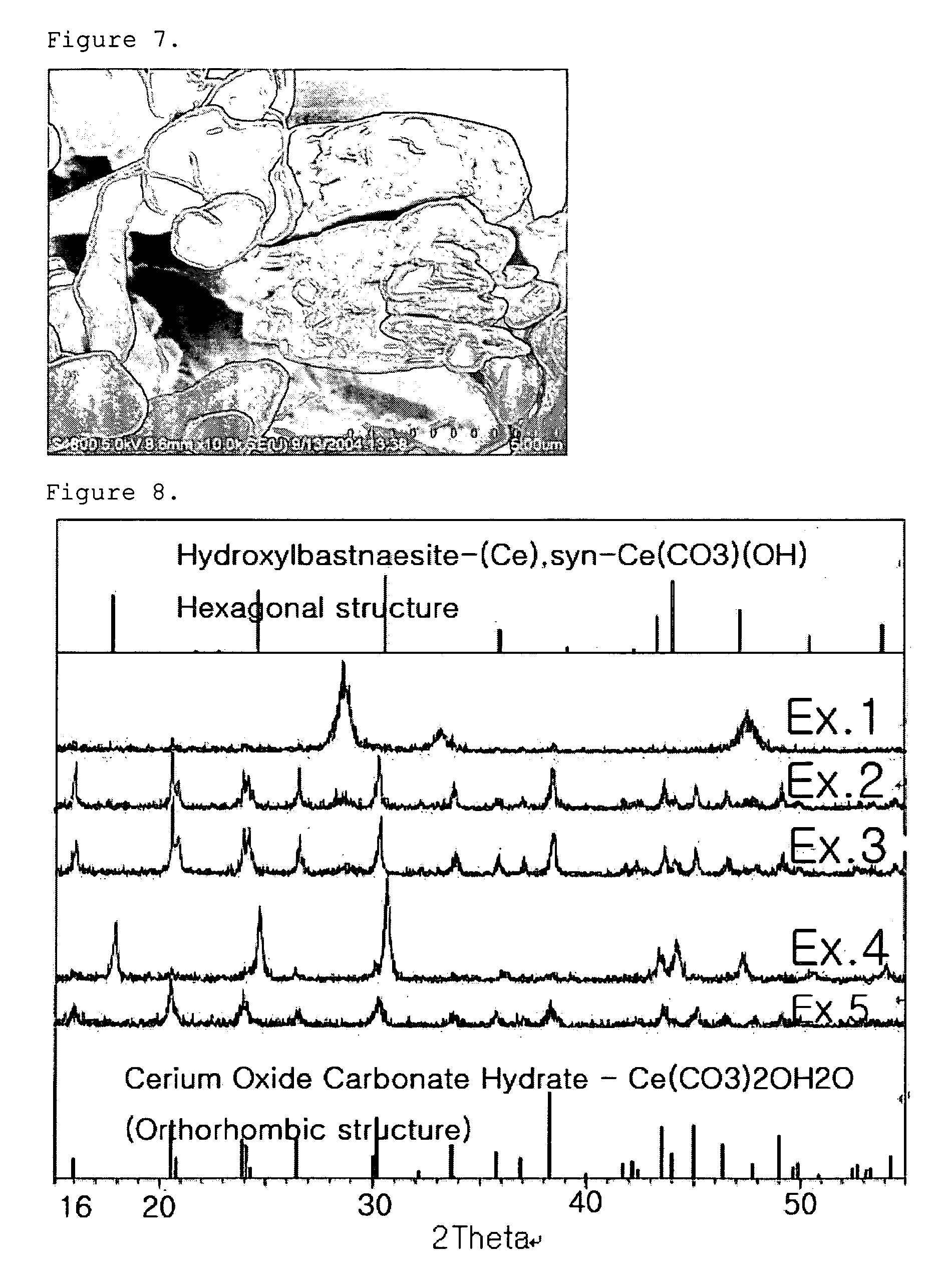
[0057] First, 0.1 mole of cerium nitrate anhydrate was dissolved into 100 ml of distilled water, and the solution was stirred at room temperature for 30 minutes. In a separate container, 0.2 mole of ammonium carbonate was dissolved into 100 ml of ethanol at 50° C. Then, the above two solutions were mixed with each other to cause precipitation at 75° C. for 6 hours. By doing so, cerium carbonate powder was obtained.
[0058] The resultant cerium carbonate powder had a cubic structure and a uniform size of 100 nm. The particle shape is shown in FIG. 1 by using SEM at a magnification of ×20,000 (herein, each scale bar has a length of 2 μm). Additionally, crystallinity of the resultant powder is shown in FIG. 8 by using XRD.
example 2
[0059] Cerium carbonate powder was obtained in the same manner as described in Example 1, except that 1,4-butanediol was used instead of ethanol and the reaction was performed at a temperature of 85° C. instead of 75° C.
[0060] The resultant cerium carbonate powder (cerium oxide carbonate hydrate-Ce(CO3)2O.H2O) had an orthorhombic structure and a size of about 2˜3 μm. The particle shape is shown in FIG. 2 by using SEM at a magnification of ×10,000 (herein, each scale bar has a length of 5 μm). Additionally, crystallinity of the resultant powder is shown in FIG. 8 by using XRD.
example 3
[0061] Cerium carbonate powder was obtained in the same manner as described in Example 1, except that ethylene glycol was used instead of ethanol and the reaction was performed at a temperature of 85° C. instead of 75° C.
[0062] The resultant cerium carbonate powder (cerium oxide carbonate hydrate-Ce(CO3)2O.H2O) had an orthorhombic structure and a size of about 500˜600 nm. The particle shape is shown in FIG. 3 by using SEM at a magnification of ×20,000 (herein, each scale bar has a length of 2 μm). Additionally, crystallinity of the resultant powder is shown in FIG. 8 by using XRD.
[0063] In the above Example 1, the reaction was performed at 75° C., which was lower than the boiling point of ethanol. As can be seen from Examples 1˜3, it is possible to vary the size and shape of cerium carbonate particles by varying the organic solvent used in the cerium precursor and carbonate precursor.
PUM
| Property | Measurement | Unit |
|---|---|---|
| dielectric constant | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com