Process for the direct production of methanol from methane
a technology of methanol and methane, which is applied in the field of converting methane to methanol, can solve the problems of low thermodynamic and kinetic stability of methane, low utilization rate of methane as a chemical feedstock, and relatively high transportation cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
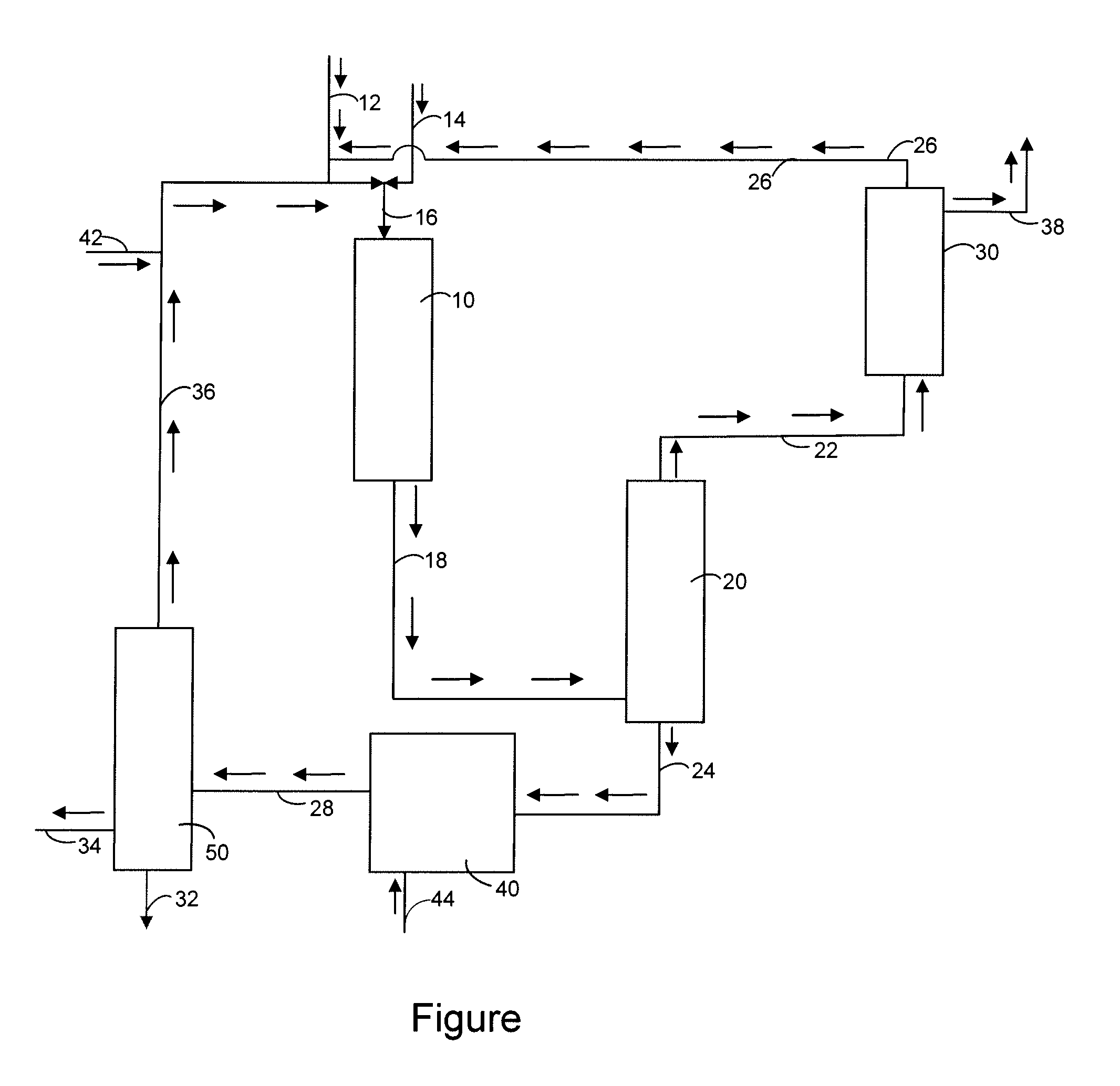
[0013]The present invention relates to the selective oxidation of methane to methanol using a catalyst. The methane or feedstream can either be a pure methane stream or can be diluted with an inert gas such as nitrogen, helium, neon, argon, etc. Another element of the invention is an oxidant which will react with the methane. The oxidant can be oxygen, hydrogen peroxide or an organic hydroperoxide. Non limiting examples of organic hydroperoxides include tert-butyl hydroperoxide, cumene hydroperoxide, etc. It is also within the scope of the invention that a blend of hydrocarbons such as gasoline, straight run diesel, light cycle oil, vacuum gas oil, fuel oil and crude oil can be oxidized to give a mixture of organic hydroperoxides. One process for producing these hydroperoxides is disclosed in U.S. Pat. No. 7,038,090 B1 which is incorporated by reference in its entirety.
[0014]The oxidant stream and methane are now flowed into an oxidation reactor or oxidation zone where they are reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com